The Wittig Reaction
- Page ID
- 5823
Organophosphorus ylides react with aldehydes or ketones to give substituted alkenes in a transformation called the Wittig reaction. This reaction is named for George Wittig who was awarded the Nobel prize for this work in 1979. A principal advantage of alkene synthesis by the Wittig reaction is that the location of the double bond is absolutely fixed, in contrast to the mixtures often produced by alcohol dehydration.
Preparation of Phosphorus Ylides
It has been noted that dipolar phosphorus compounds are stabilized by p-d bonding. This bonding stabilization extends to carbanions adjacent to phosphonium centers, and the zwitterionic conjugate bases derived from such cations are known as ylides. An ylide is defined as a compound with opposite charges on adjacent atoms both of which have complete octets. For the Wittig reaction discussed below an organophosphorus ylide, also called Wittig reagents, will be used. The ability of phosphorus to hold more than eight valence electrons allows for a resonance structure to be drawn forming a double bonded structure.
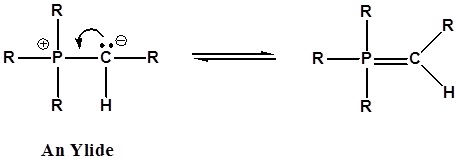
The stabilization of the carbanion provided by the phosphorus causes an increase in acidity (pKa ~35). Very strong bases, such as butyl lithium, are required for complete formation of ylides.
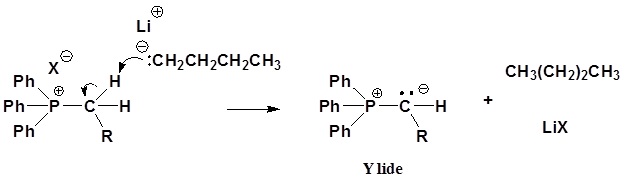
The ylides shown here are all strong bases. Like other strongly basic organic reagents, they are protonated by water and alcohols, and are sensitive to oxygen. Water decomposes phosphorous ylides to hydrocarbons and phosphine oxides, as shown.
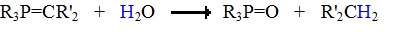
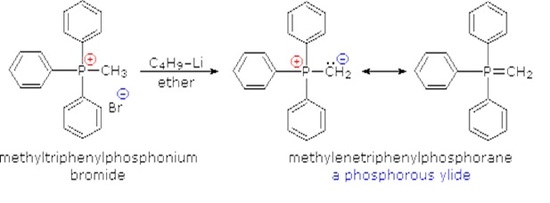
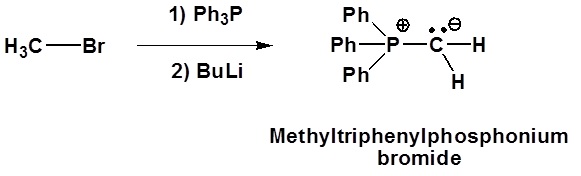
Although many ylides are commercially available it is often necessary to create them synthetically. Ylides can be synthesized from an alkyl halide and a trialkyl phosphine. Typically triphenyl phosphine is used to synthesize ylides. Because a SN2 reaction is used in the ylide synthesis methyl and primary halides perform the best. Secondary halides can also be used but the yields are generally lower. This should be considered when planning out a synthesis which involves a synthesized Wittig reagent.

Mechanism of ylide formation
1) SN2 reaction
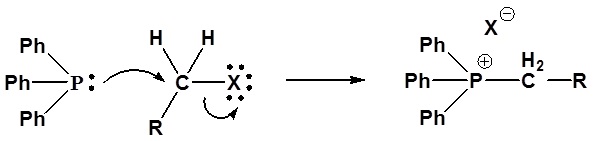
2) Deprotonation
Examples of ylide formation
The Wittig Reaction
The most important use of ylides in synthesis comes from their reactions with aldehydes and ketones, which are initiated in every case by a covalent bonding of the nucleophilic alpha-carbon to the electrophilic carbonyl carbon. Ylides react to give substituted alkenes in a transformation called the Wittig reaction. This reaction is named for George Wittig who was awarded the Nobel prize for this work in 1979. A principal advantage of alkene synthesis by the Wittig reaction is that the location of the double bond is absolutely fixed, in contrast to the mixtures often produced by alcohol dehydration.
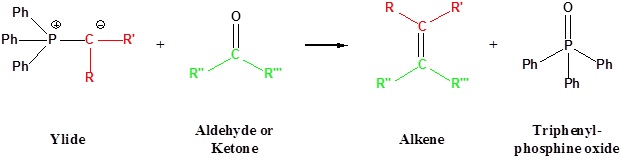
Going from reactants to products simplified
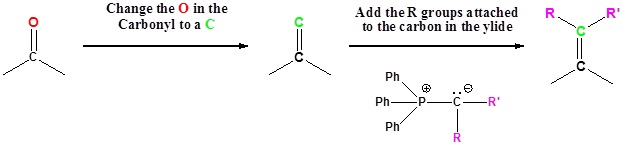
Examples of the Wittig reaction
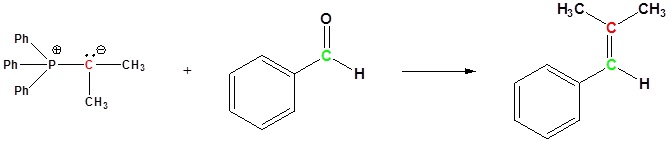
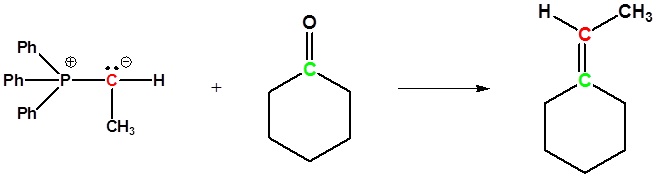
Mechanism of the Wittig reaction
Following the initial carbon-carbon bond formation, two intermediates have been identified for the Wittig reaction, a dipolar charge-separated species called a betaine and a four-membered heterocyclic structure referred to as an oxaphosphatane. Cleavage of the oxaphosphatane to alkene and phosphine oxide products is exothermic and irreversible.
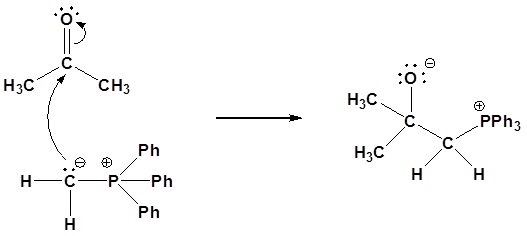
1) Nucleophillic attack on the carbonyl
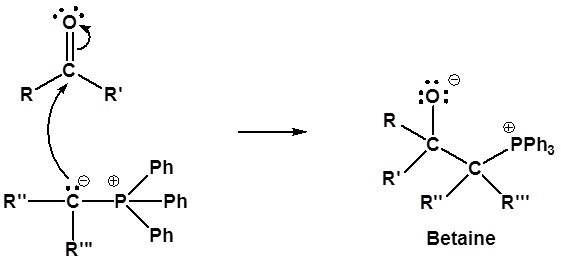
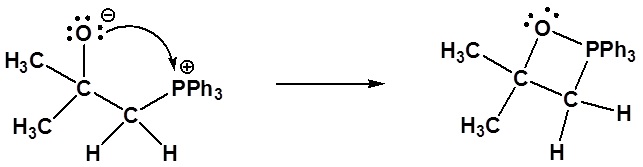
2) Formation of a 4 membered ring
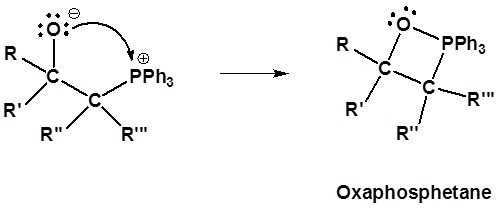
3) Formation of the alkene
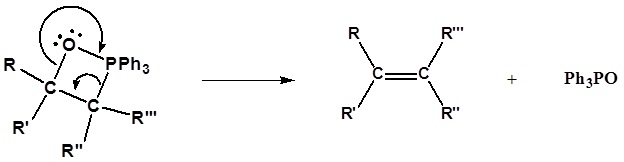
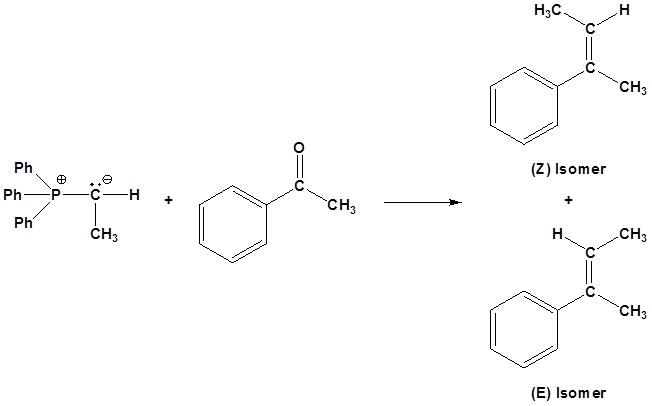
Limitation of the Wittig reaction
If possible both E and Z isomer of the double bond will be formed. This should be considered when planning a synthesis involving a Wittig Reaction.
Problems
1) Please write the product of the following reactions.
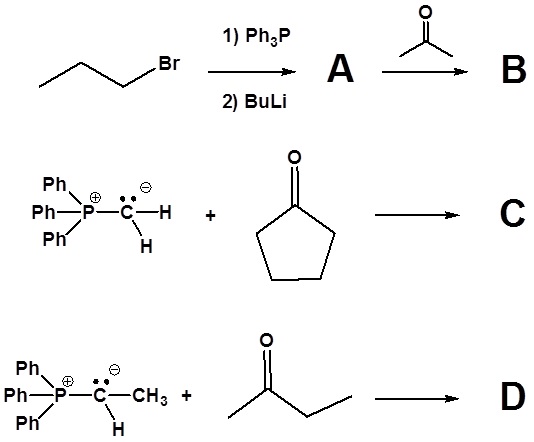
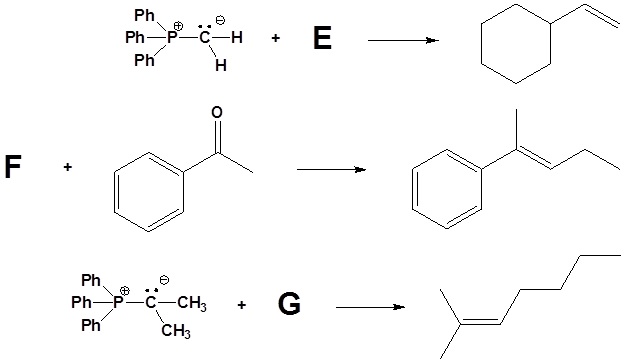
2) Please indicate the starting material required to produce the product.
3) Please draw the structure of the oxaphosphetane which is made during the mechanism of the reaction given that produces product C.
4) Please draw the structure of the betaine which is made during the mechanism of the reaction given that produces product D.
5) Please give a detailed mechanism and the final product of this reaction
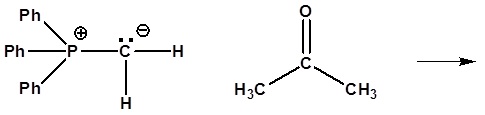
6) It has been shown that reacting and epoxide with triphenylphosphine forms an alkene. Please propose a mechanism for this reaction. Review the section on epoxide reactions if you need help.
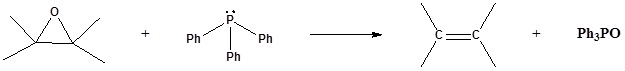
Answers
1)
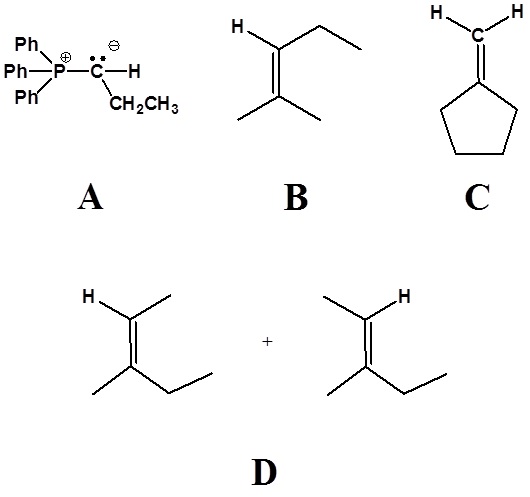
2)
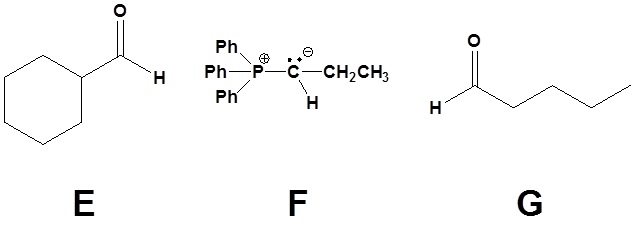
3)
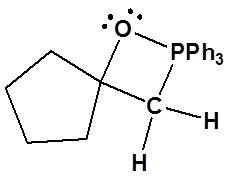
4)
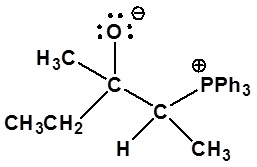
5)
Nucleophillic attack on the carbonyl
Formation of a 4 membered ring
Formation of the alkene
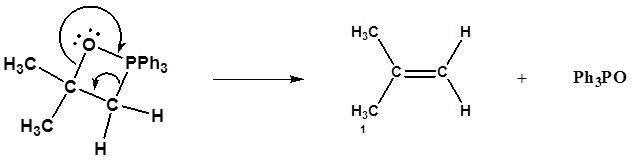
6) Nucleophillic attack on the epoxide
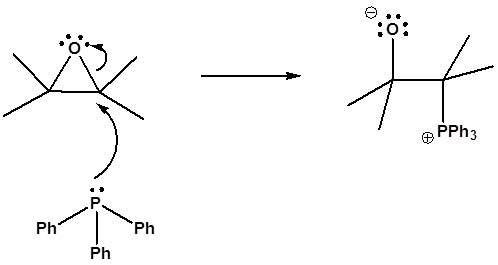
Formation of a 4 membered ring
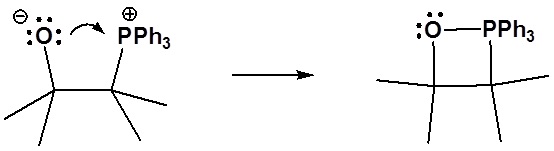
Formation of the alkene
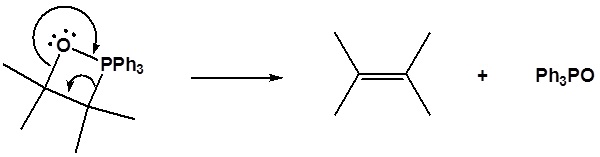
Contributors
Prof. Steven Farmer (Sonoma State University)
William Reusch, Professor Emeritus (Michigan State U.), Virtual Textbook of Organic Chemistry

