Grignard and Organolithium Reagents
- Page ID
- 5736
The alkali metals (Li, Na, K etc.) and the alkaline earth metals (Mg and Ca, together with Zn) are good reducing agents, the former being stronger than the latter. These same metals reduce the carbon-halogen bonds of alkyl halides. The halogen is converted to a halide anion, and the carbon bonds to the metal which has characteristics similar to a carbanion (R:-).
Formation of Organometallic Reagents
Many organometallic reagents are commercially available, however, it is often necessary to make then. The following equations illustrate these reactions for the commonly used metals lithium and magnesium (R may be hydrogen or alkyl groups in any combination).
- An Alkyl Lithium Reagent
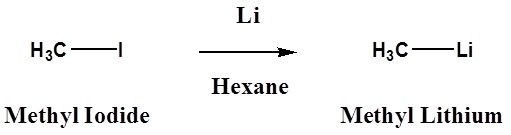
\[ \ce{R3C-X} + \ce{2Li} \rightarrow \ce{R3C-Li} + \ce{LiX}\]
- A Grignard Regent
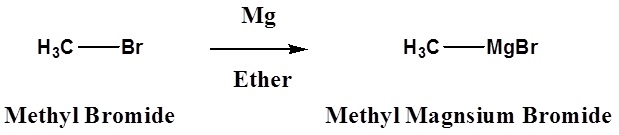
\[\ce{R3C-X} + \ce{Mg} \rightarrow \ce{R3C-MgX}\]
Halide reactivity in these reactions increases in the order: Cl < Br < I and Fluorides are usually not used. The alkyl magnesium halides described in the second reaction are called Grignard Reagents after the French chemist, Victor Grignard, who discovered them and received the Nobel prize in 1912 for this work. The other metals mentioned above react in a similar manner, but Grignard and Alky Lithium Reagents most widely used. Although the formulas drawn here for the alkyl lithium and Grignard reagents reflect the stoichiometry of the reactions and are widely used in the chemical literature, they do not accurately depict the structural nature of these remarkable substances. Mixtures of polymeric and other associated and complexed species are in equilibrium under the conditions normally used for their preparation.
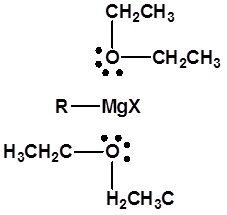
A suitable solvent must be used. For alkyl lithium formation pentane or hexane are usually used. Diethyl ether can also be used but the subsequent alkyl lithium reagent must be used immediately after preparation due to an interaction with the solvent. Ethyl ether or THF are essential for Grignard reagent formation. Lone pair electrons from two ether molecules form a complex with the magnesium in the Grignard reagent (As pictured below). This complex helps stabilize the organometallic and increases its ability to react.
These reactions are obviously substitution reactions, but they cannot be classified as nucleophilic substitutions, as were the earlier reactions of alkyl halides. Because the functional carbon atom has been reduced, the polarity of the resulting functional group is inverted (an originally electrophilic carbon becomes nucleophilic). This change, shown below, makes alkyl lithium and Grignard reagents excellent nucleophiles and useful reactants in synthesis.
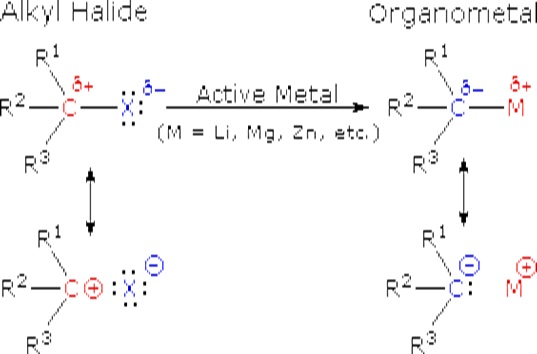
Common Organometallic Reagents
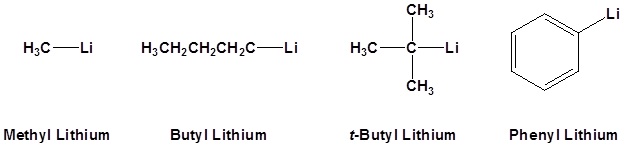
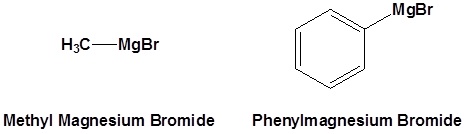
Reaction of Organometallic Reagents with Various Carbonyls
Because organometallic reagents react as their corresponding carbanion, they are excellent nucleophiles. The basic reaction involves the nucleophilic attack of the carbanionic carbon in the organometallic reagent with the electrophilic carbon in the carbonyl to form alcohols.
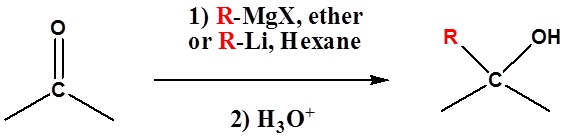
Both Grignard and Organolithium Reagents will perform these reactions.
Addition to formaldehyde gives 1° alcohols
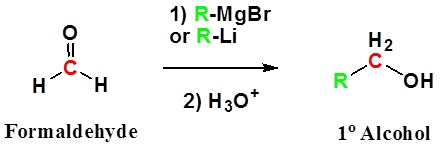
Addition to aldehydes gives 2° alcohols
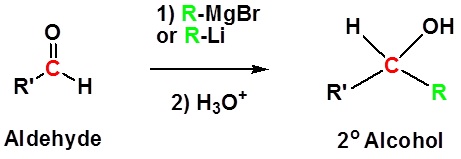
Addition to ketones gives 3° alcohols
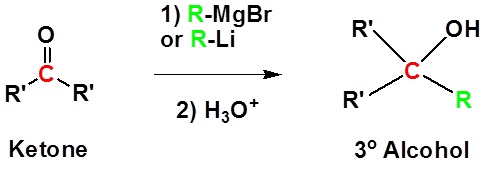
Addition to carbon dioxide (CO2) forms a carboxylic acid
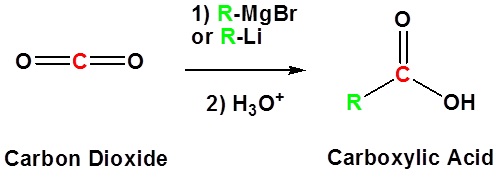
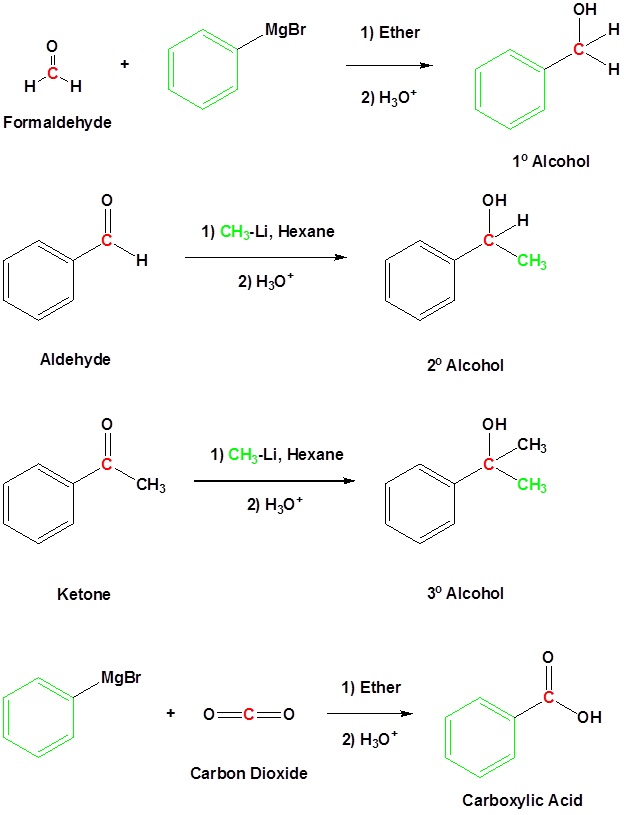
Going from Reactants to Products Simplified
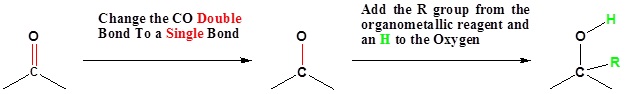
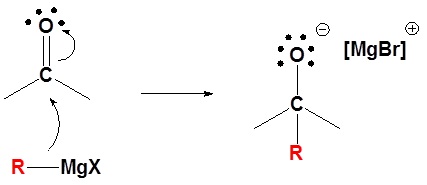
Mechanism for the Addition to Carbonyls
The mechanism for a Grignard agent is shown; the mechanism for an organometallic reagent is the same.
1) Nucleophilic attack
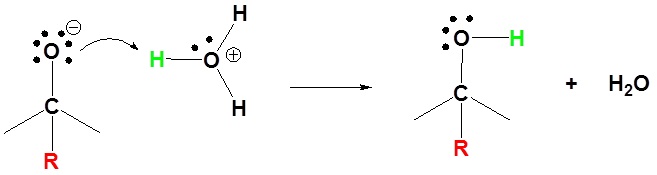
2) Protonation
Organometallic Reagents as Bases
These reagents are very strong bases (pKa's of saturated hydrocarbons range from 42 to 50). Although not usually done with Grignard reagents, organolithium reagents can be used as strong bases. Both Grignard reagents and organolithium reagents react with water to form the corresponding hydrocarbon. This is why so much care is needed to insure dry glassware and solvents when working with organometallic reagents.

In fact, the reactivity of Grignard reagents and organolithium reagents can be exploited to create a new method for the conversion of halogens to the corresponding hydrocarbon (illustrated below). The halogen is converted to an organometallic reagent and then subsequently reacted with water to from an alkane.
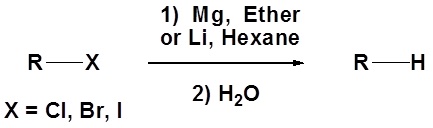
Limitation of Organometallic Reagents
As discussed above, Grignard and organolithium reagents are powerful bases. Because of this they cannot be used as nucleophiles on compounds which contain acidic hydrogens. If they are used they will act as a base and deprotonate the acidic hydrogen rather than act as a nucleophile and attack the carbonyl. A partial list of functional groups which cannot be used are: alcohols, amides, 1o amines, 2o amines, carboxylic acids, and terminal alkynes.
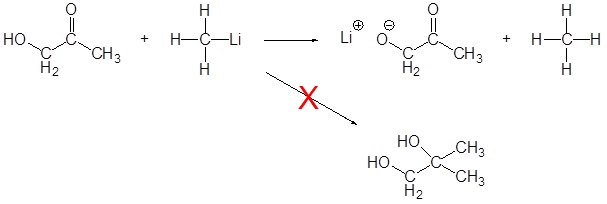
Problems
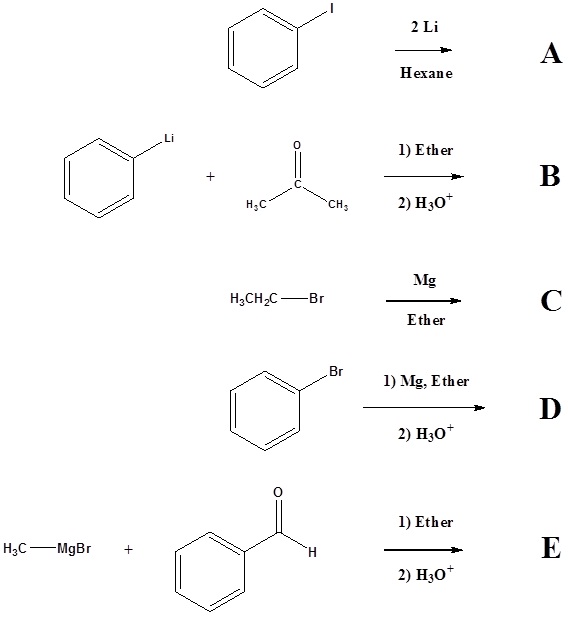
1) Please write the product of the following reactions.
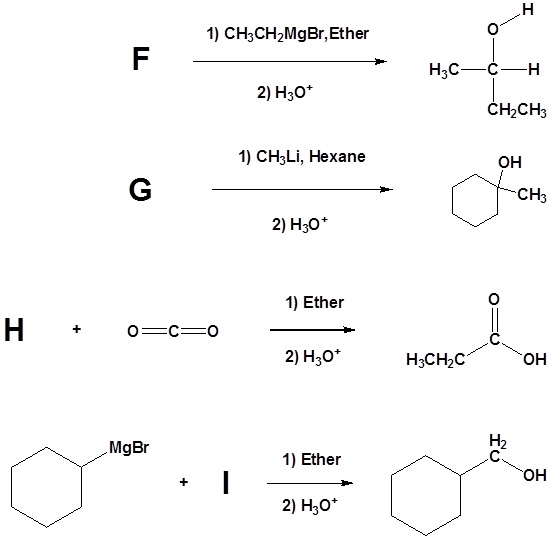
2) Please indicate the starting material required to produce the product.
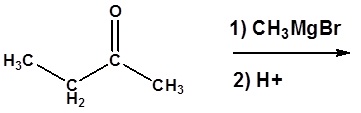
3) Please give a detailed mechanism and the final product of this reaction
4) Please show two sets of reactants which could be used to synthesize the following molecule using a Grignard reaction.
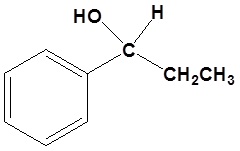
Answers
1)
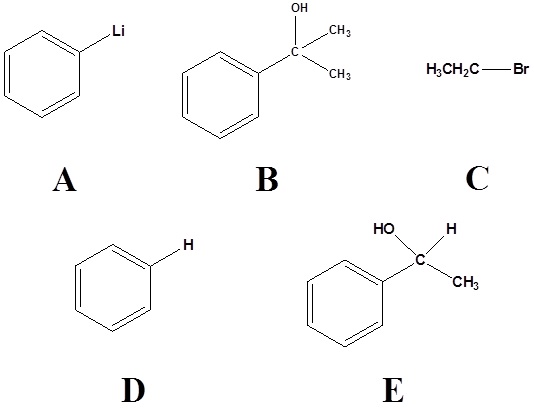
2)
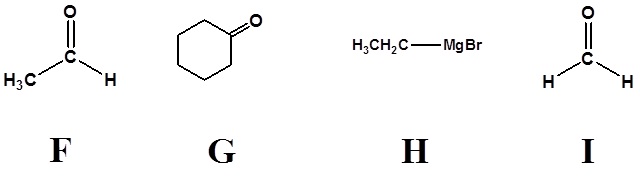
3)
Nucleophilic attack
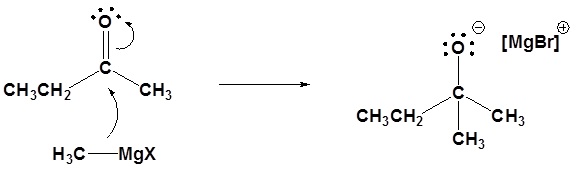
Protonation
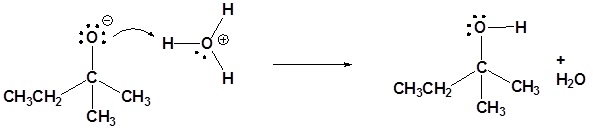
4)
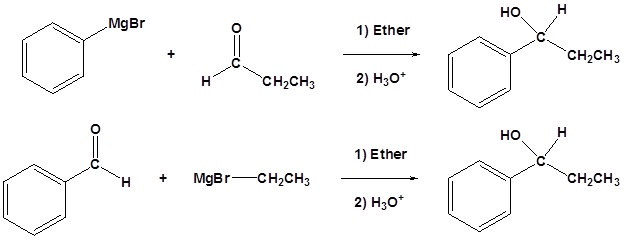
Contributors
Prof. Steven Farmer (Sonoma State University)
William Reusch, Professor Emeritus (Michigan State U.), Virtual Textbook of Organic Chemistry

