II. Intramolecular Addition of Carbon-Centered Radicals to Aldehydo and Keto Groups
- Page ID
- 24054
The possibility of isolating a product from intermolecular addition of a carbon-centered radical to an aldehyde or ketone is small due to the ready reversibility of this reaction (eq 1), but the possibility of product isolation increases considerably if the reaction becomes an intramolecular addition of a carbon-centered radical to an aldehydo or keto group to give a radical centered on an oxygen atom that is attached to a five- or six-membered ring.
An example of such a reaction is shown in Scheme 1, where the carbon-centered radical 2, generated from 6-bromohexanal (1), is converted reversibly into the cyclic alkoxy radical 3.1 Hydrogen-atom abstraction by 3 from tri-n-butyltin hydride has a substantially larger rate constant than that for abstraction by 2; consequently, even though ring opening is more rapid than ring closure, reaction produces cyclohexanol as the major product and hexanal as a minor one.
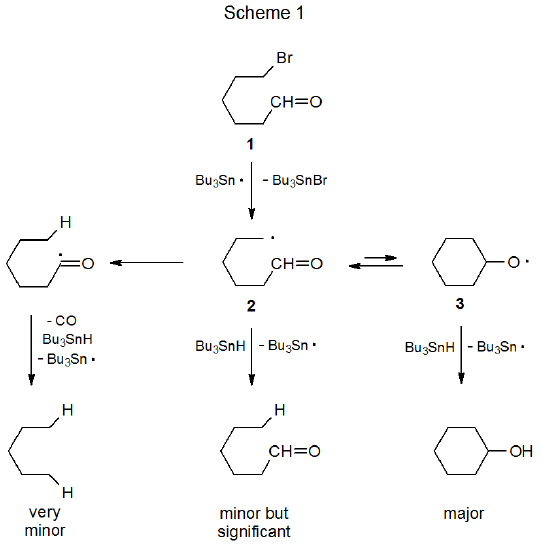
Intramolecular hydrogen-atom abstraction from the aldehydo group in 2 is a very minor process. The inability of this abstraction to compete with ring formation in a noncarbohydrate system is echoed in the reactions of carbohydrate radicals containing aldehydo groups. The reaction shown in Scheme 2 is one of several discussed in this chapter where hydrogen-atom abstraction from an aldehydo group is possible but does not take place.2
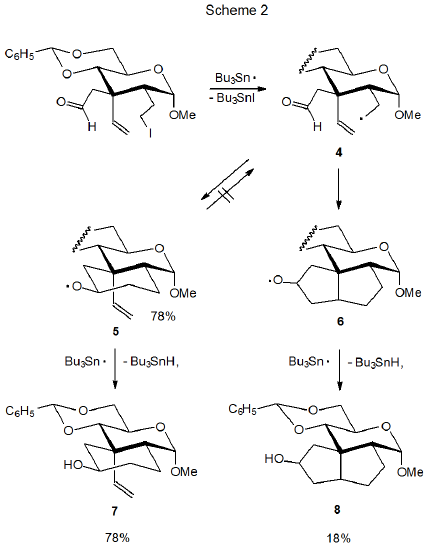
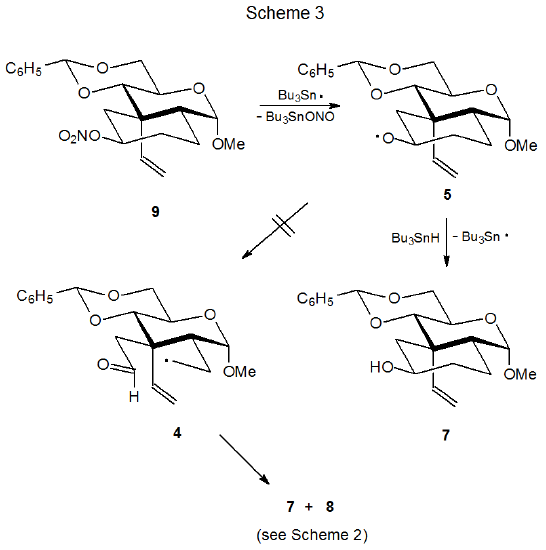
Even though ring opening always is a possibility for cyclic alkoxy radicals, this transformation sometimes does not take place; for example, the reaction producing the alkoxy radical 5 from the ring-open radical 4 is not reversible (Scheme 2).2,3 Failure of the cyclohexane ring in 5 to open is demonstrated by reaction of the nitrate ester 9 (Scheme 3).3 Treatment of 9 with Bu3SnH produces 5 (and ultimately the product 7) but ring opening to give 4 does not happen. If the ring-open radical 4 were formed, the product 8 also would be produced in this reaction, but since no 8 could be detected, the conclusion is that the alkoxy radical 5 does not undergo ring opening.3
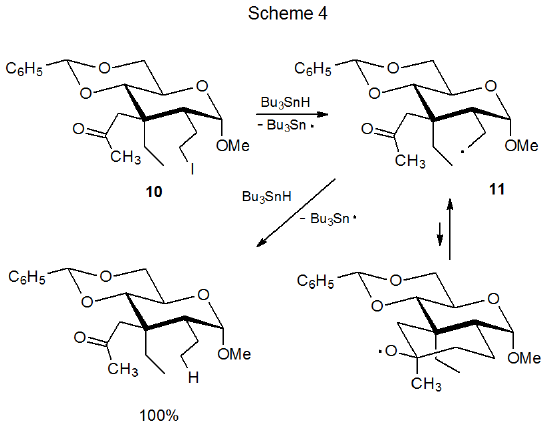
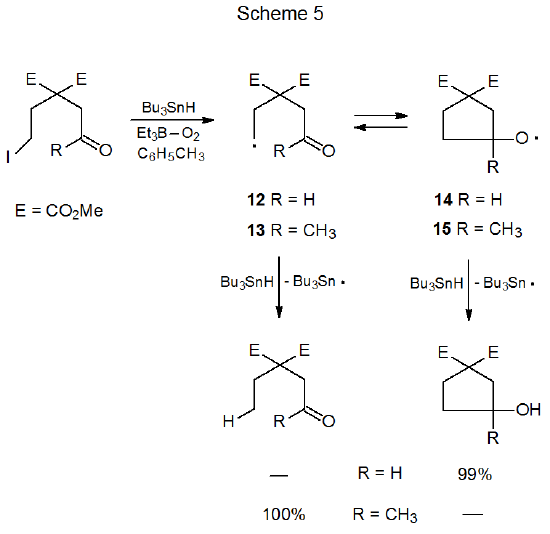
In contrast to cyclization of the aldehydo radical 4 (Scheme 2) the closely related keto radical 11 (Scheme 4) does not form a new ring system.4 Either the greater steric hindrance inherent in producing a tertiary alkoxy radical or rapid ring opening of such a strained intermediate or both are sufficient to prevent 11 from forming a new ring system. These reasons for failure to form a new ring draw support from the reactions of noncarbohydrate radicals 13 and 14 (Scheme 5).5 In the reaction shown in Scheme 5 where R is a methyl group, hydrogen-atom abstraction from tri-n-butyltin hydride is done exclusively by the open-chain radical 13. When R is a hydrogen atom, abstraction from Bu3SnH occurs only after conversion of the open-chain radical 12 into the cyclic alkoxy radical 14.
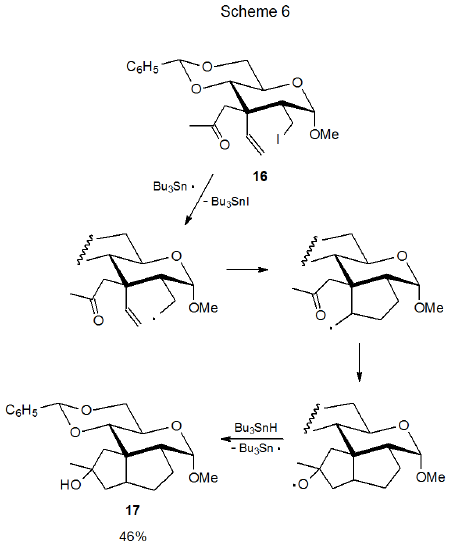
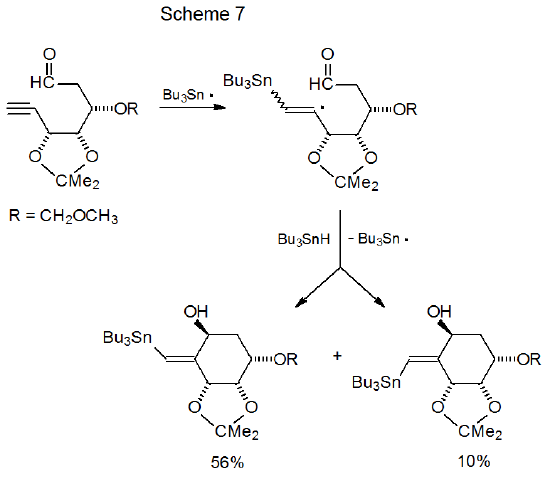
The reactivates of the aldehydo radical 4 (Scheme 2) and the keto radical 11 (Scheme 4) raise a number of questions (listed below) about participation of keto and aldehydo groups in radical cyclization reactions. Many of these questions have been answered by study of related compounds. Their answers provide insight into the factors that control the cyclization process. These questions and their answers are:
.png?revision=1&size=bestfit&width=435&height=118)
.png?revision=1&size=bestfit&width=315&height=106)
.png?revision=1&size=bestfit&width=425&height=313)
Roger W. Binkley (Cleveland State University) and Edith R. Binkley (Cleveland Heights-University Heights school system)

