IV. Addition of Tin- and Silicon-Centered Radicals to Aldehydes
- Page ID
- 24056
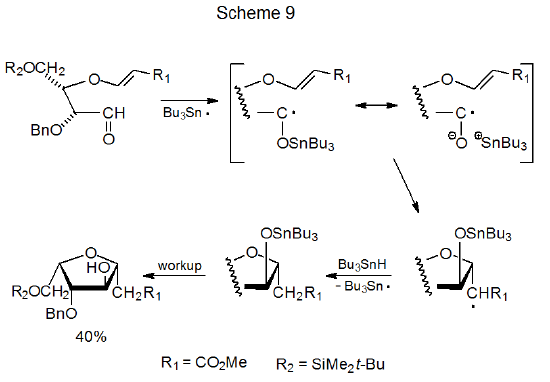
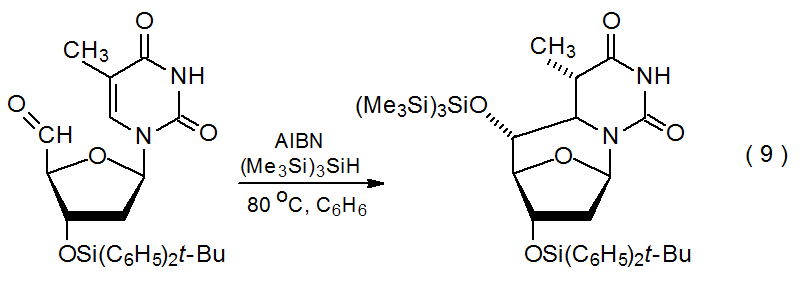
Although the reactions discussed thus far have involved addition of carbon-centered radicals to carbonyl groups, other types of radicals, including tin- and silicon-centered ones, also add to aldehydes and ketones. Reaction of the tri-n-butyltin radical with a carbonyl group generates a tin ketyl, a radical with considerable negative charge on the oxygen atom. As the reaction in Scheme 918 shows, tin ketyls undergo internal radical addition to electron-deficient, C–C multiple bonds.18–22 These ketyls also react with C–N double bonds,23 and they produce pinacols upon addition to carbonyl groups (eq 8).24 Internal addition also can occur when a silicon-centered radical adds to an aldehydo group, as happens in the reaction shown in eq 9.25
.png?revision=1&size=bestfit&width=290&height=243)
.png?revision=1&size=bestfit&width=405&height=142)

