VI. Ketone Photolysis
- Page ID
- 24058
A. α-Cleavage Reactions
When photolysis of ketones causes homolytic cleavage of a bond between the carbonyl group and one of the α carbon atoms either a pair of radicals or a diradical forms. (Such a reaction is known as an α-cleavage or Norrish Type I reaction.) α-Cleavage of simple ketones does not take place in solution, although it does occur in the gas phase. Cleavage in solution happens only when stabilized radicals are produced. This means that the reaction shown in eq 3 is successful for nonvolatile compounds such as carbohydrates only when the radical center in R· is stabilized in some way (e.g., by having an oxygen or nitrogen atom attached). Most carbohydrates that contain a keto group will have at least one pathway for forming an oxygen-stabilized radical by α-cleavage.54
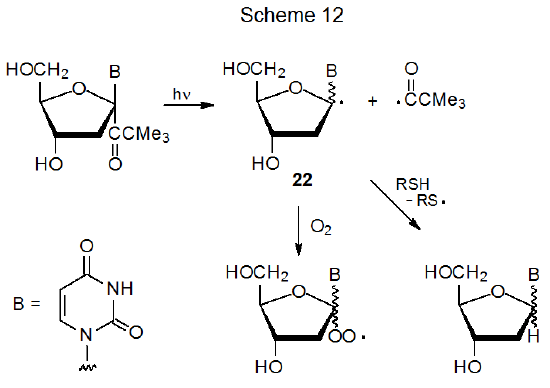
When the keto group in a carbohydrate is not part of a ring system, α‑cleavage produces a radical pair. Most reactions of this type involve derivatives of nucleosides, nucleotides, or oligonucleotides.55–67 Scheme 12 describes such a reaction, one in which the nucleoside member (22) of the radical pair produced by α-cleavage undergoes two characteristic radical reactions, namely, hydrogen-atom abstraction (when an effective donor, such as a thiol, is present) and addition of O2 (when molecular oxygen is one of the reactants).61
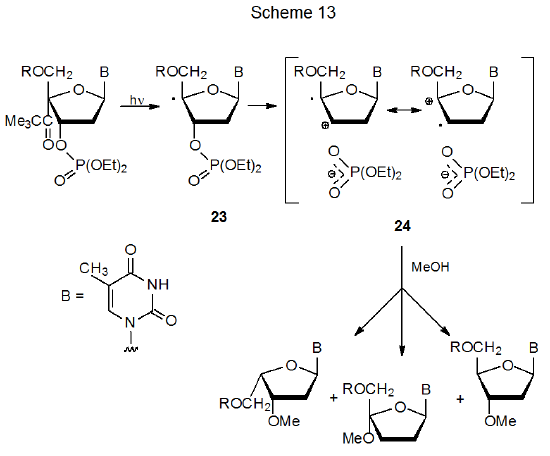
Although α-cleavage in nucleotides produces radicals that undergo typical radical reactions, such as those shown in Scheme 12, many of these radicals also undergo a heterolytic cleavage to form radical cations and phosphate anions. An example of such a reaction is shown in Scheme 13, where the radical 23 cleaves its C-3'–O bond to generate the radical cation 24 and a phosphate anion.56 (Radical-cation formation of the type shown in Scheme 13 is discussed in Section III of Chapter 9.)
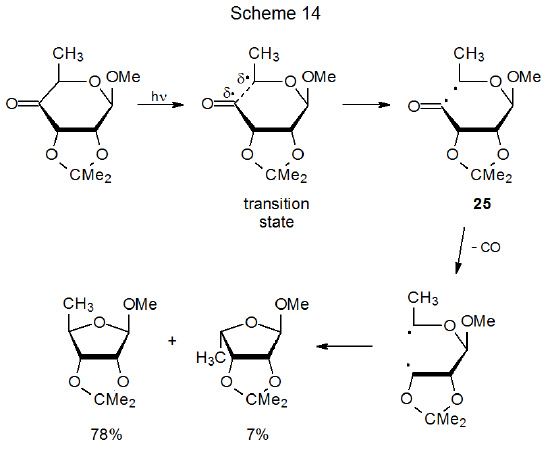
α-Cleavage in a cyclic ketone, a reaction that occurs in many carbohydrates, is an internal process that produces a diradical.68 Diradicals of this type usually reform a ring system, but often after the loss of carbon monoxide.68 Scheme 14 describes formation of the diradical 25, a reaction that is followed by loss of carbon monoxide to give a second diradical, one that produces a new ring system by radical combination.69 The α-cleavage shown in Scheme 14 is driven, at least in part, by transition-state stabilization due to the developing radical center at C-6 being stabilized by an attached oxygen atom.
B. Hydrogen-Atom Abstraction Reactions
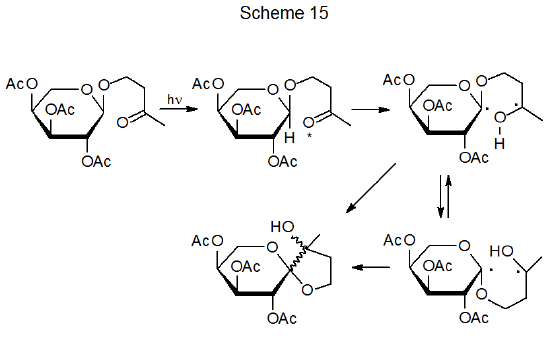
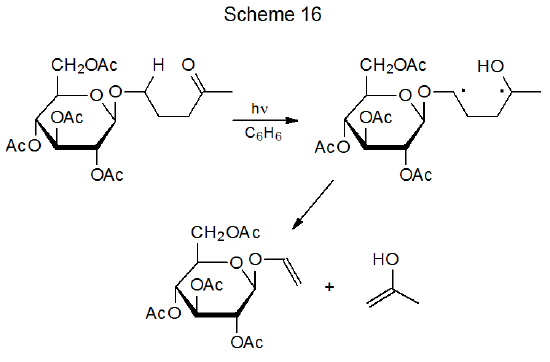
Ketones that do not undergo α-cleavage have another option for diradical formation, namely, internal abstraction that occurs when a hydrogen atom comes with bonding distance of an excited carbonyl group; thus, in the reaction is shown in Scheme 15, 1,6-hydrogen-atom abstraction produces a diradical that then forms a spiro compound by radical combination.70 If a 1,5-hydrogen-atom transfer takes place, the resulting 1,4-diradical fragments as shown in Scheme 16.71 (Many carbohydrates undergo this type of reaction, which is known as a Norrish Type II process.72)
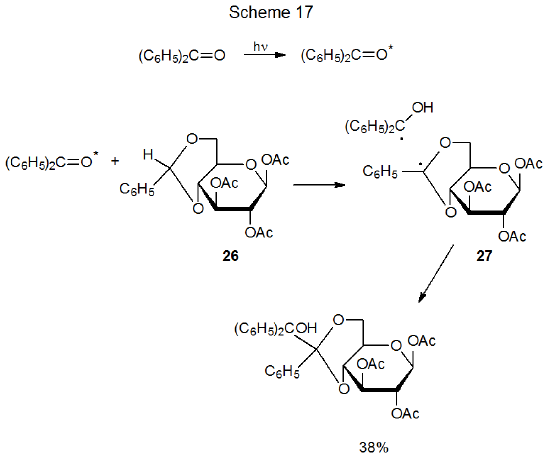
If an excited ketone does not undergo internal abstraction or α-cleavage, hydrogen-atom abstraction from another molecule sometimes takes place.73 Such abstraction requires a transition state in which there is considerable radical stabilization. Hydrogen-atom abstraction by excited benzophenone from the benzylidene acetal 26 meets this requirement by producing the highly stabilized radical 27 (Scheme 17).74 Radical combination completes this reaction.