III. Nitriles
- Page ID
- 24550
A. Synthesis
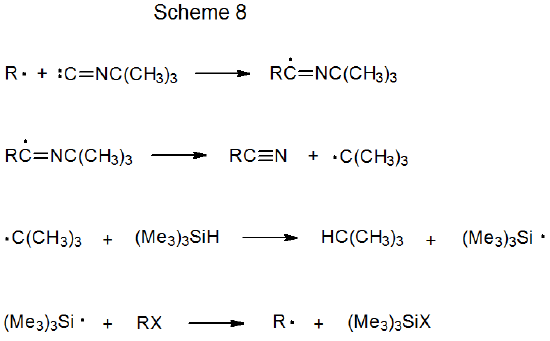
When t-butyl isonitrile reacts with a carbon-centered radical, an addition-elimination process takes place that results in the formation of a nitrile (Scheme 8).16–23 This type of nitrile synthesis is illustrated by the reaction shown in eq 5.20 (Scheme 10 in Chapter 2 describes another example of this type of reaction.22)
.png?revision=1&size=bestfit&width=420&height=126)
B. Reactions
1. Addition to a Cyano Group
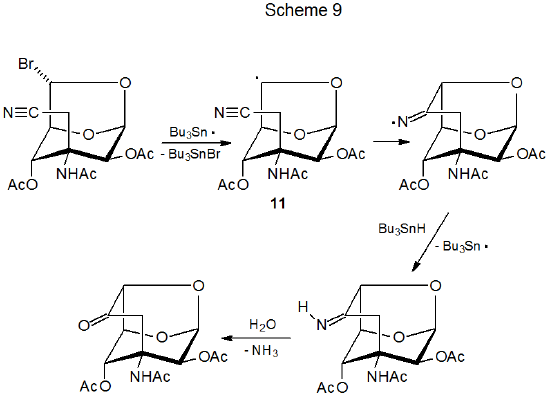
Radical reactions of carbohydrate nitriles usually involve internal addition in which a carbon-centered radical generated in close proximity to a cyano group forms a new ring system.24–31 An example is shown in Scheme 9, where the radical centered on C-6 in 11 adds to the cyano group as a part of this sequential process.24 Carbohydrate radical formation in this type of reaction typically begins with the tri-n-butyltin radical abstracting a halogen atom or an O-thiocarbonyl group. After cyclization, the radical abstracts a hydrogen atom from Bu3SnH to produce an imine, which in some cases is isolated and in others is hydrolyzed to the corresponding carbonyl compound before isolation.
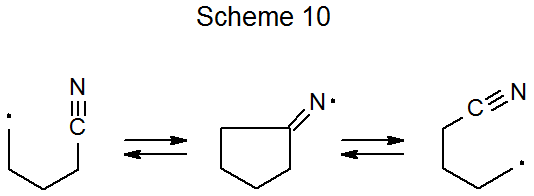
2. Cyano Group Migration
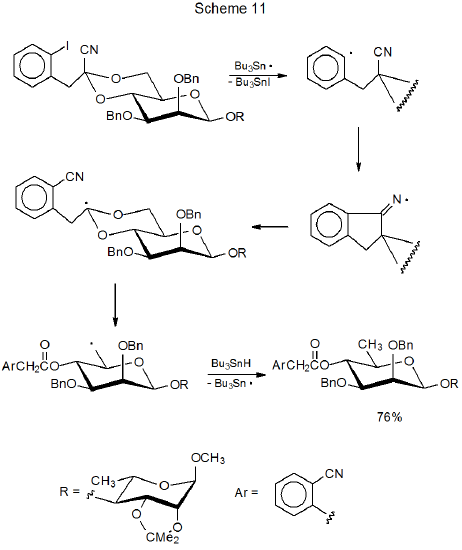
When a cyclic imino radical forms during carbon-centered radical addition to a cyano group, the possibility exists that ring opening will lead to cyano group migration (Scheme 10).32–34 Such a reaction is shown in Scheme 11, where migration accompanies ring opening of a benzylidene acetal.32