III. Hydrazones and Imines
- Page ID
- 24554
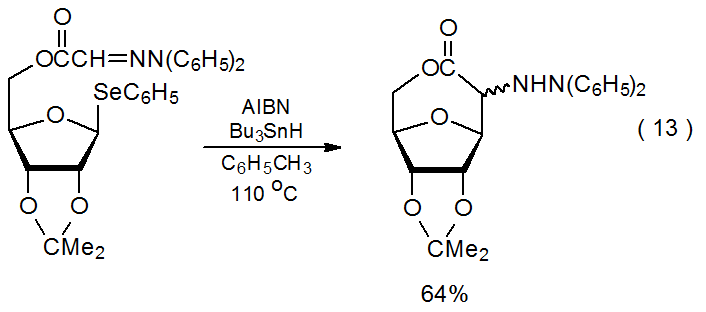
Radical reactions of carbohydrate hydrazones2,43–46 are less common than those of oxime ethers; reactions of imines43 are still more rare. The reported reactions of hydrazones, such as that shown in eq 12,43 all involve radical cyclization. The substrates in most of these reactions are esters derived from (2,2-diphenylhydrazono)acetic acid (eq 13).44,45
.png?revision=1&size=bestfit&width=430&height=132)
.png?revision=1&size=bestfit&width=355&height=157)
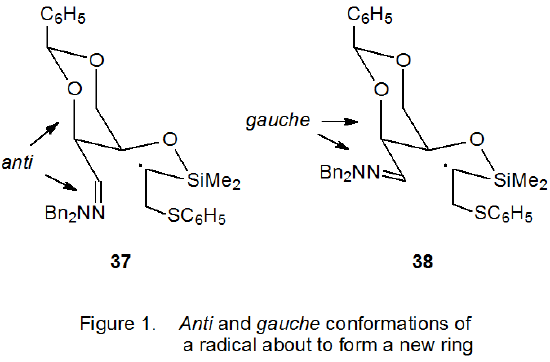
The reaction shown in eq 14 pictures a highly stereoselective cyclization involving a carbohydrate hydrazone.46 Stereoselectivity in this reaction is determined by the preferred conformation (37, Figure 1) of the intermediate produced by a phenylthiyl radical adding to the carbon–carbon double bond in the substrate. Conformation 37 has the carbon–nitrogen bond anti to the adjacent carbon–oxygen bond. The conformation 38, expected to be more stable because it has more pseudoequatorial substituents, is destabilized by dipole-dipole interactions arising from a gauche relation between the neighboring C=N and C–O bonds.46
.png?revision=1&size=bestfit&width=430&height=187)