V. Protonated Heteroaromatics
- Page ID
- 24556
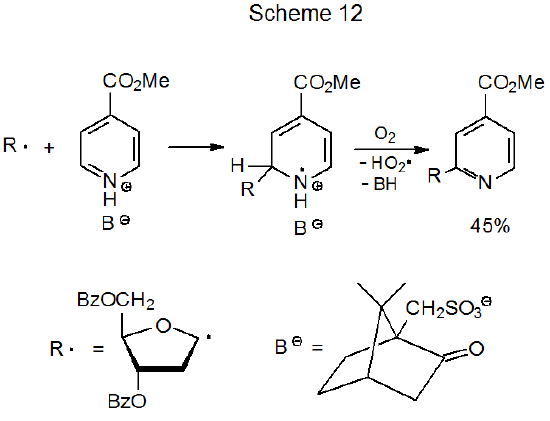
Pyranos-1-yl and furanos-1-yl radicals add to protonated heteroaromatics to produce C-nucleoside derivatives.48–54 A proposed mechanism for this type of reaction is given in Scheme 12.50 Protonation dramatically increases the rate of addition of a carbon-centered radical to a heteroaromatic compound; in fact, the rate is so fast that it is not necessary to conduct the reaction in an inert atmosphere. Not only does reaction take place in the presence of molecular oxygen but oxygen is a likely participant in the rearomatization stage of this process (Scheme 12). In reactions of this type the initially formed radical (R·) usually is generated by photolysis of an O‑acyl-N-hydroxy-2-thiopyridone (eq 15).48 (Reactions of O‑acyl-N-hydroxy-2-thiopyridones are discussed in Chapter 13.)
.png?revision=1&size=bestfit&width=385&height=245)

