III. Ammonium Cerium(IV) Nitrate
- Page ID
- 24634
A. Addition of CH-Acidic Compounds to D-Glycals
1. Dimethyl Malonate
a. Regioselectivity
In a manner similar to manganese(III) acetate reaction, ammonium cerium(IV) nitrate promotes regioselective addition of CH-acidic compounds to carbohydrates with electron-rich double bonds.6–9,15,16,19–22 Examples of such reactions are given in equations 3 and 4, and a mechanism for the addition process is proposed in Scheme 7.6–9 Reactions of glycals with (NH4)2Ce(NO3)6 (eq 3 and eq 4) can be conducted at lower temperatures than those with Mn(OAc)3 (eq 1 and eq 2). These milder conditions completely suppress formation of the Ferrier rearrangement product 8, a compound formed in the reaction given in eq 1 but absent in that shown in eq 3.
.png?revision=1&size=bestfit&width=420&height=135)
.png?revision=1&size=bestfit&width=425&height=121)
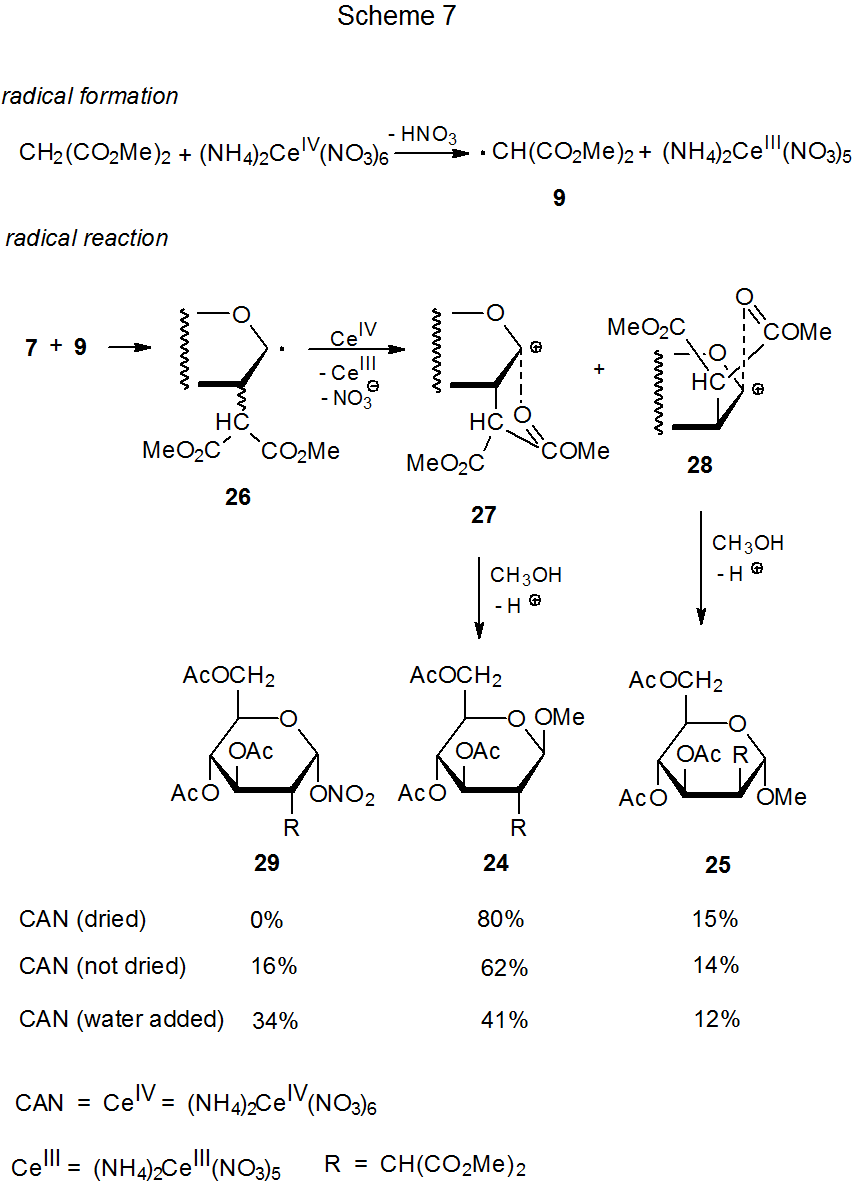
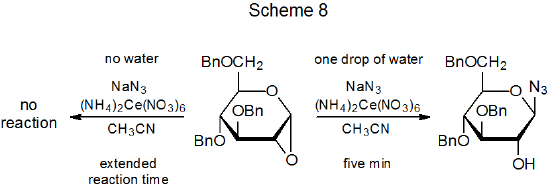
If water is present, even in small amounts, a new compound (29) is produced in the reaction shown in Scheme 7.22 How is this compound formed? Direct reaction between the cation 27 and the nitrate anion is one possibility, but if this pathway is the correct one, addition of sodium nitrate to the reaction mixture should increase the yield of 29. It does not.7 Ligand transfer from (NH4)2Ce(NO3)6 to the radical 26 also is possible,6,7 but it is difficult to see why such a process should be dependent on the amount of water present in the reaction mixture. Both of these possibilities [reaction of 27 with NaNO3 or ligand transfer from (NH4)2Ce(NO3)6] appear more likely to produce the anomer of 29 rather than 29 itself. The data in Scheme 7 do show that formation of the nitrate 29 comes at the expense of the β-glycoside 24. Conversion of 24 into 29 could result from reaction of 24 with the nitric acid produced by interaction of (NH4)2Ce(NO3)6 with water. This possibility is supported by the reaction shown in Scheme 8, where a drop of water apparently reacts with (NH4)2Ce(NO3)6 to create the nitric acid needed for an acid-catalyzed ring opening.23
b. Stereoselectivity
Reaction stereoselectivity improves when (NH4)2Ce(NO3)6 replaces Mn(OAc)3 in the addition of dimethyl malonate to 3,4,6-tri-O-acetyl-D-glucal (7). The ratio of α-face to β-face addition at C-2 by the malonyl radical changes from 52:14 (eq 1, Scheme 4)6 to 80:15 (eq 3, Scheme 7).22 The difference in the temperature of these reactions [95 oC (eq 1) to 0 oC (eq 3)] is a likely cause for this increase in stereoselectivity.
A second, stereoselective step in the reactions shown in Schemes 3 and 7 occurs during solvent capture by intermediate cations. Methanol reacts with the cations 27 and 28 exclusively from the face of the ring opposite to the malonyl group and produces a single stereoisomer in each case (Scheme 7). The capture of acetic acid pictured in Scheme 3 also is stereoselective but less so because each intermediate cation reacts to give a mixture of stereoisomers. Once again, greater reaction stereoselectivity correlates with lower reaction temperature.
Stereoselectivity in malonyl-radical addition also increases when approach to one face of a ring becomes more difficult due to a change in substrate structure. Such a change occurs when 3,4,6-tri-O-acetyl-D-glucal (7) (eq 3) is replaced by 3,4,6-tri-O-acetyl-D-galactal (30) (eq 4).7 Projection of the C-4 acetoxy group onto the β face of the pyranoid ring in 30 makes this face more congested than the β face of the pyranoid ring in 7.
c. Reactivity
(1). Effect of C-1 Substituents on Glycal Reactivity Ortho-Ester Formation
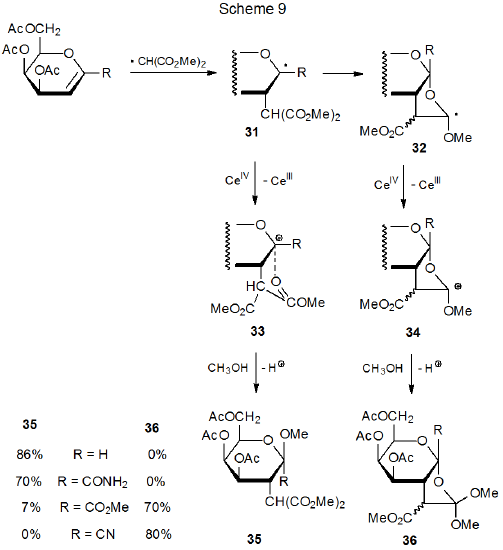
The products formed from addition of the malonyl radical 9 to C-1 substituted glycals depend on the structure of the C-1 substituents (Scheme 9).24 When R is H or C(=O)NH2, the glycoside 35 forms, but when R is CO2Me or CN, the products are the orthoesters 36. An explanation for this difference in reactivity is that when R is highly electron-withdrawing (e.g., CN or CO2Me), the oxidation potential of the radical 31 is high enough that its conversion to the cation 33 by reaction with (NH4)2Ce(NO3)6 is suppressed (Scheme 9).24,25 When this suppression occurs, cyclization of 31 produces 32, a radical that now can be oxidized easily to the corresponding cation (34). Reaction of this cation with methanol then gives the orthoesters 36.
(2). Effect of a C-3 Substituent of Glycal Reactivity
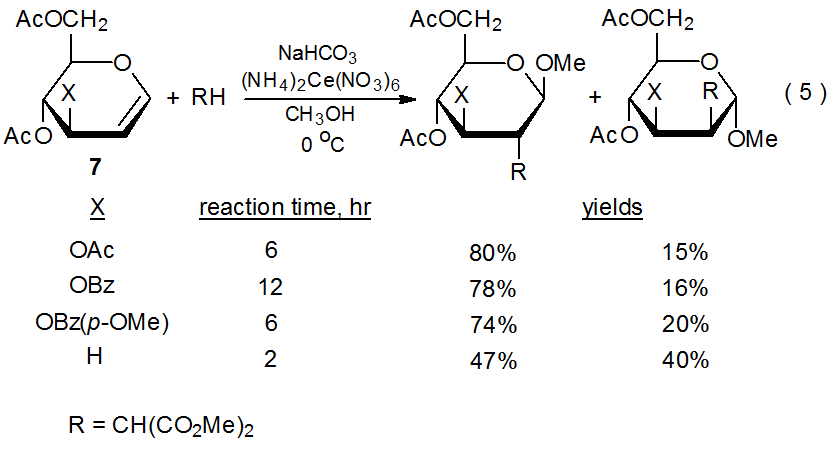
The importance of electron-withdrawing substituents to glycal reactivity also is apparent when different substituents are attached to C-3 (eq 5).25 The reactions shown in eq 5 confirm the previously mentioned findings (Sections III.A.1.a and III.A.1.b) about regioselectivity (the malonyl radical 9 adds exclusively to C-2) and stereoselectivity (9 adds preferentially to the face of the pyranoid ring opposite to that containing the C-3 substituent). These reactions also demonstrate the effect of the electron-withdrawing character of a C-3 substituent on reaction rate (eq 5). Since the reactions involve the electrophilic malonyl radical adding to an electron-rich double bond, increasing the electron-withdrawing character of the R group decreases the reaction rate by reducing the electron density in the double bond; thus, an O-benzoyl group, which is more electron-withdrawing than an O-acetyl group, causes a slower rate of reaction. The reaction rate of an O-benzoyl-substituted glycal can be increased by placing an electron-donating methoxy group in the benzene ring. Reaction can be made even faster by eliminating any electron-withdrawing group from C-3 (eq 5).
.png?revision=1&size=bestfit&width=420&height=225)
2. Ethyl Nitroacetate
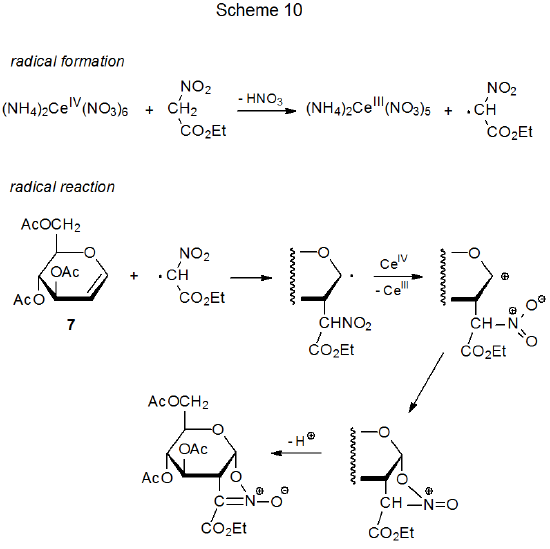
When ethyl nitroacetate reacts with (NH4)2Ce(NO3)6 in the presence of the D-glucal 7 (eq 6),20 a transformation takes place that is similar in its early stages to the dimethyl malonate reaction shown in eq 3. These two processes follow different pathways once the adduct radical has been oxidized to a cation. In the ethyl nitroacetate reaction, cyclization occurs (Scheme 10) rather than the solvent capture that characterizes reaction with dimethyl malonate (Scheme 7).
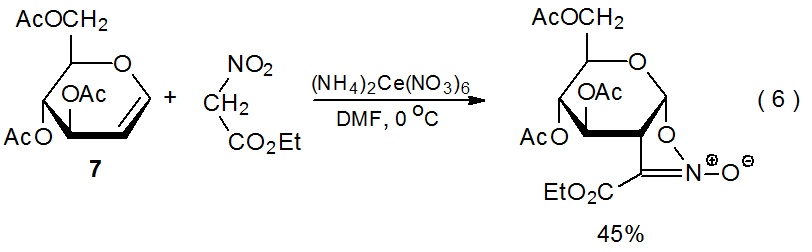.png?revision=1&size=bestfit&width=410&height=127)
3. Nitromethane
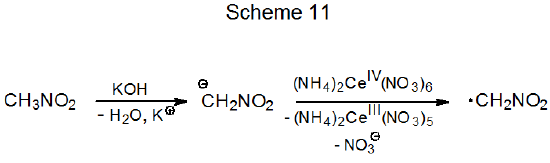
Nitromethane is a CH-acidic compound that reacts with potassium hydroxide to form a nitronate anion. Oxidation of this anion with ammonium cerium(IV) nitrate produces the electrophilic radical ·CH2NO2 (Scheme 11). If a compound with an electron-rich double bond is present in the reaction mixture, radical addition takes place (eq 7).26
.png?revision=1&size=bestfit&width=430&height=112)
B. Addition of the Azide Radical to a D-Glycal
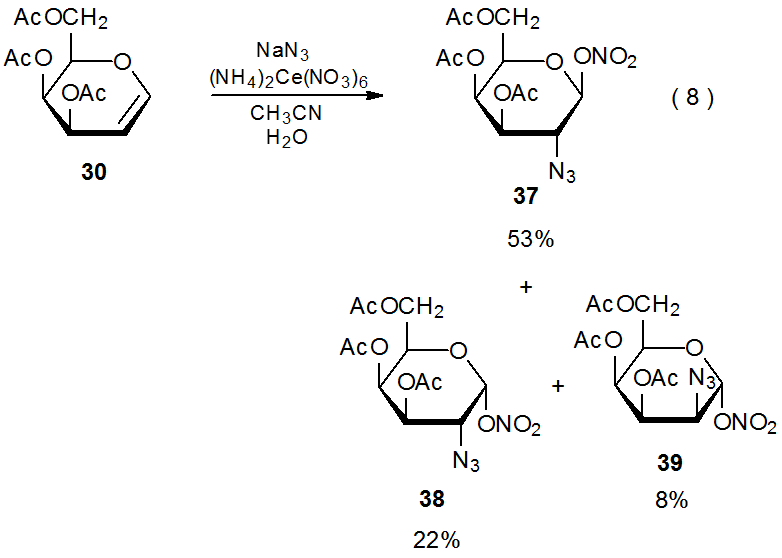
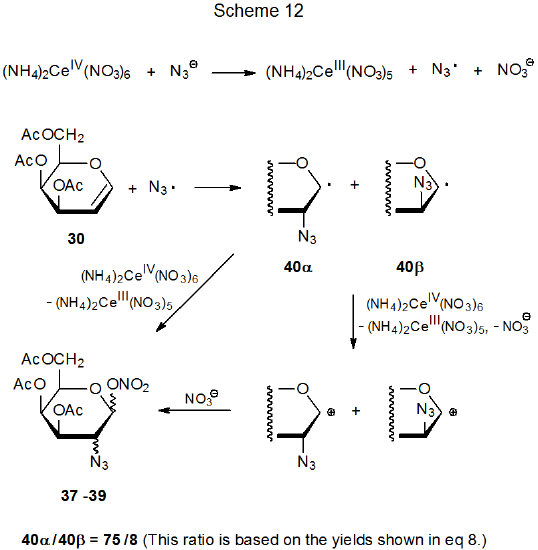
Reaction of ammonium cerium(IV) nitrate with sodium azide in the presence of the D-galactal 30 produces the diastereomeric azido nitrates 37-39 (eq 8).27 There is convincing evidence that (NH4)2Ce(NO3)6 oxidizes NaN3 to produce the azide radical (Scheme 12).28 Highly stereoselective addition of this radical to 30 gives adduct radicals 40α and 40βin a ratio of 75:8. The azido nitrates 37-39 then form either indirectly by reaction of nitrate ion with the cations produced by oxidation of 40α and 40β or directly by ligand transfer from (NH4)2Ce(NO3)6 to these radicals.29 (Section II.B.2 of Chapter 15 contains more information on azidonitration and additional references to this reaction.)
.png?revision=1&size=bestfit&width=395&height=282)
C. Addition of a Phosphonyl Radical to a D-Glycal
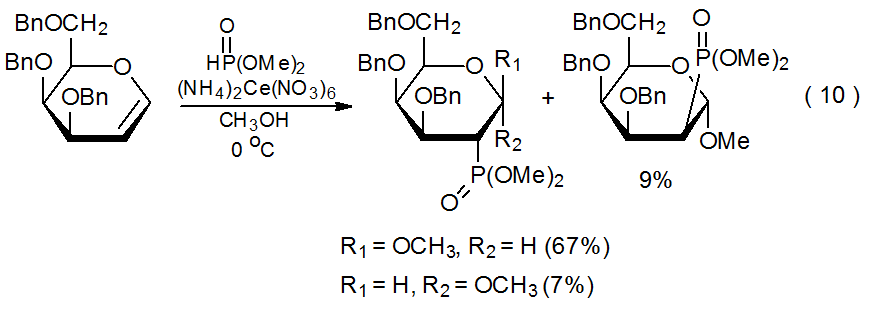
Dimethyl phosphite reacts with (NH4)2Ce(NO3)6 to produce the phosphorous-centered radical 41 (eq 9).30 This radical then adds to D-glycals in a regiospecific, highly stereoselective manner (eq 10).
.png?revision=1&size=bestfit&width=435&height=42)
.png?revision=1&size=bestfit&width=440&height=156)

