I. Organocobalt Compounds
- Page ID
- 24650
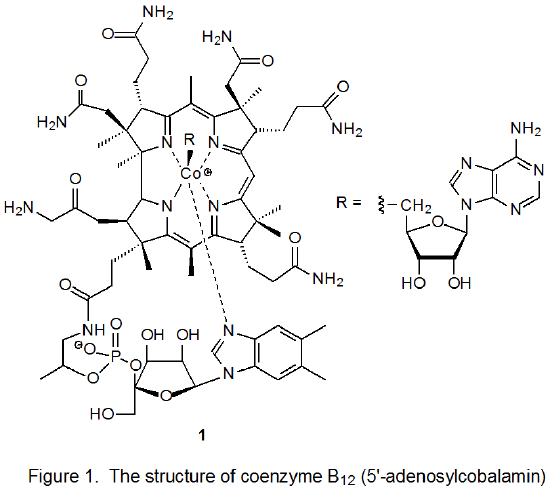
An organometallic complex that contains a carbon–cobalt bond can function as a radical precursor because such a bond is easily broken homolytically. Facile cleavage occurs because carbon–cobalt bonds are significantly weaker than most covalent bonds:1,2 in fact, the C–Co bond in coenzyme B12 (1, Figure 1) is one of the weakest covalent bonds known (BDE = 31.5 kcal mol‑1).3 Enzymatic reaction, mild heating, and photolysis with visible light all cause homolysis of C–Co bonds. Adding to the usefulness of organocobalt complexes as radical precursors is the fact that, despite their considerable reactivity, many of these complexes can be handled in the laboratory.
Although C–Co bond homolysis takes place at relatively low temperatures, photolysis is the method of choice for radical formation in reactions conducted outside biological settings.4–9 The reason for this choice is that C–Co bond fragmentation occurs with low-energy (visible) light at temperatures that avoid possible side reactions from even mild heating of complex, cobalt-containing compounds.
Coenzyme B12 (1, Figure 1) provided the original stimulus for using carbon–cobalt bond homolysis to form carbon-centered radicals.7–11 The enzyme-induced homolysis of the C–Co bond in 1 produced the 5‑deoxyadenosyl radical 2 and the cobalt-containing radical 3 (eq 1). The discovery that carbon-centered radicals could be produced in this way led to interest in finding simpler molecules that would mimic such behavior.
.png?revision=1&size=bestfit&width=430&height=223)
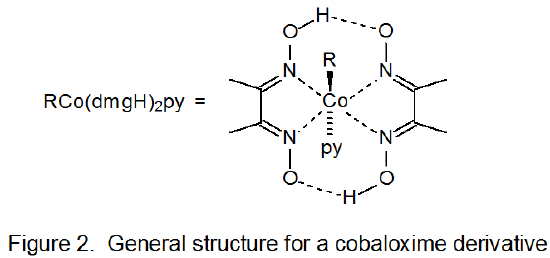
Among the several types of organocobalt complexes found to be useful in generating carbon-centered radicals, cobaloximes [bis(dimethylglyoximato)cobalt complexes] (Figure 2) are the most widely used in carbohydrate chemistry.11-15 Many reactions of cobalt-containing carbohydrates and much of the mechanistic information about reactions caused by C–Co bond homolysis come from study of cobaloximes.