II. Organomercury Compounds
- Page ID
- 24651
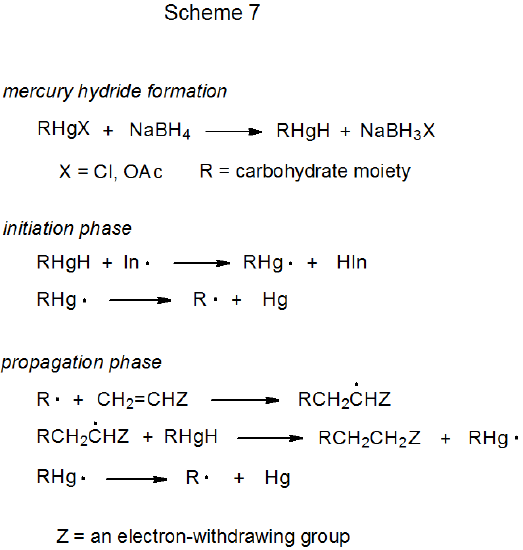
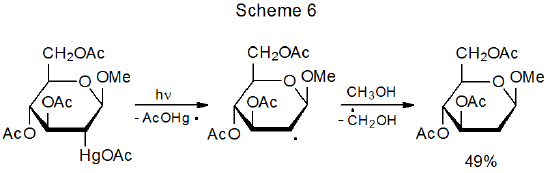
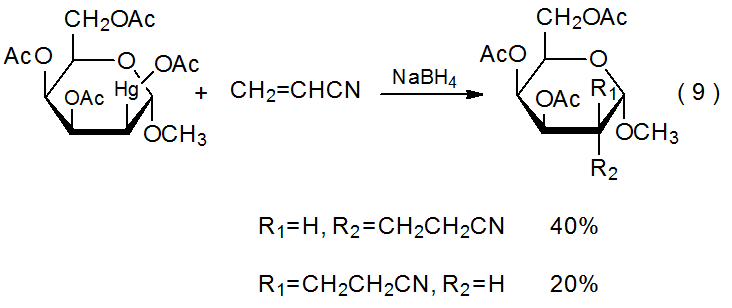
There are similarities between the reactivities of carbon–cobalt and carbon–mercury bonds. Both are strong enough to exist in stable structures that can be isolated and both readily cleave upon heating or photolysis. The result in each case is formation of a metal-centered and a carbon-centered radical. Carbon-centered radicals produced by carbon–mercury bond homolysis undergo typical radical reactions, such as hydrogen-atom abstraction (Scheme 627),27,28 addition to a multiple bond (eq 9),29 and combination with molecular oxygen (eq 1030).30,31 Although organomercury compounds can be effective sources of carbon-centered radicals, their use in this role is limited by toxicity and environmental concerns.
.png?revision=1&size=bestfit&width=370&height=156)
.png?revision=1&size=bestfit&width=285&height=96)
Two basic methods exist for generating radicals from organomercury compounds. The first, photochemical homolysis of a carbon–mercury bond, is illustrated by the reaction shown in Scheme 6.27 The second is more complicated and consists of initially converting an organomercury compound into the corresponding mercury hydride by reaction with NaBH4 (Scheme 7).32 The hydride then produces a carbon-centered radical capable of reactions such as the addition to acrylonitrile shown in eq 9.29 Adventitious initiation is credited with beginning this reaction.