7.2: Designing a Sampling Plan
- Page ID
- 5568
A sampling plan must support the goals of an analysis. For example, a material scientist interested in characterizing a metal’s surface chemistry is more likely to choose a freshly exposed surface, created by cleaving the sample under vacuum, than a surface previously exposed to the atmosphere. In a qualitative analysis, a sample does not need to be identical to the original substance, provided that there is sufficient analyte to ensure its detection. In fact, if the goal of an analysis is to identify a trace-level component, it may be desirable to discriminate against major components when collecting samples. For an interesting discussion of the importance of a sampling plan, see Buger, J. et al. “Do Scientists and Fishermen Collect the Same Size Fish? Possible Implications for Exposure Assessment,” Environ. Res. 2006, 101, 34–41.
For a quantitative analysis, the sample’s composition must accurately represent the target population, a requirement that necessitates a careful sampling plan. Among the issues to consider are these five questions.
- From where within the target population should we collect samples?
- What type of samples should we collect?
- What is the minimum amount of sample for each analysis?
- How many samples should we analyze?
- How can we minimize the overall variance for the analysis?
7.2.1 Where to Sample the Target Population
A sampling error occurs whenever a sample’s composition is not identical to its target population. If the target population is homogeneous, then we can collect individual samples without giving consideration to where to sample. Unfortunately, in most situations the target population is heterogeneous. Due to settling, a medication available as an oral suspension may have a higher concentration of its active ingredients at the bottom of the container. The composition of a clinical sample, such as blood or urine, may depend on when it is collected. A patient’s blood glucose level, for instance, changes in response to eating and exercise. Other target populations show both a spatial and a temporal heterogeneity. The concentration of dissolved O2 in a lake is heterogeneous due both to the changing seasons and to point sources of pollution.
Note
The composition of a homogeneous target population is the same regardless of where we sample, when we sample, or the size of our sample. For a heterogeneous target population, the composition is not the same at different locations, at different times, or for different sample sizes.
If the analyte’s distribution within the target population is a concern, then our sampling plan must take this into account. When feasible, homogenizing the target population is a simple solution—in most cases, however, this is impracticable. Additionally, homogenization destroys information about the analyte’s spatial or temporal distribution within the target population, information that may be of importance.
Random Sampling
The ideal sampling plan provides an unbiased estimate of the target population’s properties. A random sampling is the easiest way to satisfy this requirement.3 Despite its apparent simplicity, a truly random sample is difficult to collect. Haphazard sampling, in which samples are collected without a sampling plan, is not random and may reflect an analyst’s unintentional biases.
Here is a simple method for ensuring that we collect random samples. First, we divide the target population into equal units and assign a unique number to each unit. Then, we use a random number table to select the units to sample. Example 7.3 provides an illustrative example.
Note
Appendix 14 provides a random number table that you can use for designing sampling plans.
Example 7.3
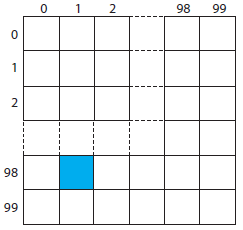
To analyze a polymer’s tensile strength, individual samples of the polymer are held between two clamps and stretched. In evaluating a production lot, the manufacturer’s sampling plan calls for collecting ten 1 cm × 1 cm samples from a 100 cm × 100 cm polymer sheet. Explain how we can use a random number table to ensure that our samples are random.
Solution
As shown by the grid, we divide the polymer sheet into 10 000 1 cm × 1 cm squares, each identified by its row number and its column number, with numbers running from 0 to 99. For example, the blue square is in row 98 and column 1. To select ten squares at random, we enter the random number table in Appendix 14 at an arbitrary point, and let the entry’s last four digits represent the row and the column for the first sample. We then move through the table in a predetermined fashion, selecting random numbers until we have 10 samples. For our first sample, let’s use the second entry in the third column, which is 76831. The first sample, therefore, is row 68 and column 31. If we proceed by moving down the third column, then the 10 samples are as follows:
| Sample | Number | Row | Column | Sample | Number | Row | Column |
|
1 2 3 5 |
76831 66558 33266 12032 14063 |
68 65 32 20 40 |
31 58 66 32 63 |
6 7 8 9 10 |
41701 38605 64516 13015 12138 |
17 86 45 30 21 |
01 05 16 15 38 |
In collecting a random sample we make no assumptions about the target population, making this the least biased approach to sampling. On the other hand, a random sample often requires more time and expense than other sampling strategies because we need a greater number of samples to ensure that we adequately sample the target population.4
Judgmental Sampling
The opposite of random sampling is selective, or judgmental sampling in which we use prior information about the target population to help guide our selection of samples. Judgmental sampling is more biased than random sampling, bur requires fewer samples. Judgmental sampling is useful if we wish to limit the number of independent variables influencing our results. For example, if we are studying the bioaccumulation of PCB’s in fish, we may choose to exclude fish that are too small or that appear diseased.
Systematic Sampling
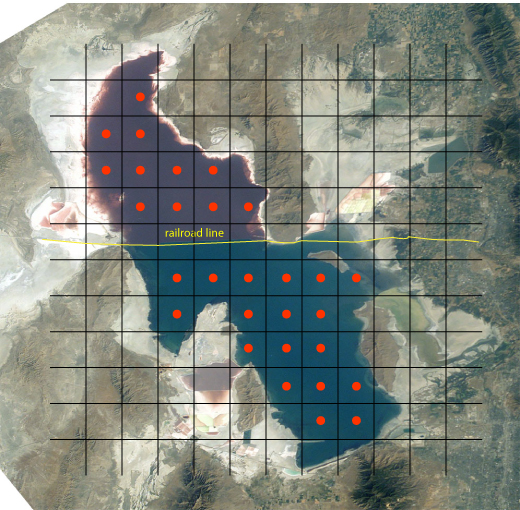
Random sampling and judgmental sampling represent extremes in bias and in the number of samples needed to characterize the target population. Systematic sampling falls in between these extremes. In systematic sampling we sample the target population at regular intervals in space or time. Figure 7.3 shows an aerial photo of the Great Salt Lake in Utah. A railroad line divides the lake into two sections with different chemical compositions. To compare the lake’s two sections—and to evaluation spatial variations within each section—we use a two-dimensional grid to define sampling locations. When a population’s heterogeneity is time-dependent, as is common in clinical studies, samples are drawn at regular intervals in time.
Figure 7.3 Aerial photo of the Great Salt Lake in Utah, taken from the International Space Station at a distance of approximately 380 km. The railroad line divides the lake into two sections that differ in chemical composition. Superimposing a two-dimensional grid divides each section of the lake into sampling units. The red dots at the center of each unit represent sampling sites. Photo courtesy of the Image Science and Analysis Laboratory, NASA Johnson Space Center, Photo Number ISS007-E-13002 (eol.jsc.nasa.gov).
If a target population’s properties have a periodic trend, a systematic sampling will lead to a significant bias if our sampling frequency is too small. This is a common problem when sampling electronic signals where the problem is known as aliasing. Consider, for example, a signal consisting of a simple sign wave. Figure 7.4a shows how an insufficient sampling frequency underestimates the signal’s true frequency. The apparent signal, shown by the dashed red line passing through the five data points, is significantly different from the true signal shown by the solid blue line.
According to the Nyquist theorem, to accurately determine a periodic signal’s true frequency, we must sample the signal at least twice during each cycle or period. If we collect samples at an interval of ∆t, the highest frequency we can accurately monitor is (2∆t)–1. For example, if our sampling rate is 1 sample/hr, the highest frequency we can monitor is (2×1 hr)–1 or 0.5 hr–1, corresponding to a period of less than 2 hr. If our signal’s period is less than 2 hours (a frequency of more than 0.5 hr–1), then we must use a faster sampling rate. Ideally, the sampling rate should be at least 3-4 times greater than the highest frequency signal of interest. If our signal has a period of one hour, we should collect a new sample every 15-20 minutes.
Figure 7.4 Effect of sampling frequency when monitoring a periodic signal. Individual samples are shown by the red dots (•). In (a) the sampling frequency is approximately 1.5 samples per period. The dashed red line shows the apparent signal based on five samples and the solid blue line shows the true signal. In (b) a sampling frequency of approximately 5 samples per period accurately reproduces the true signal.
Systematic–Judgmental Sampling
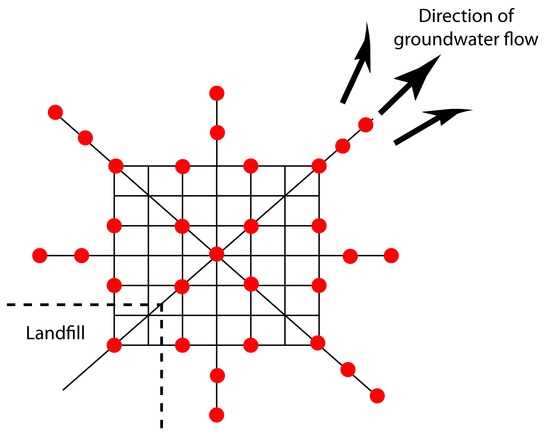
Combinations of the three primary approaches to sampling are also possible.5 One such combination is systematic–judgmental sampling, in which we use prior knowledge about a system to guide a systematic sampling plan. For example, when monitoring waste leaching from a landfill, we expect the plume to move in the same direction as the flow of groundwater—this helps focus our sampling, saving money and time. The systematic–judgmental sampling plan in Figure 7.5 includes a rectangular grid for most of the samples and linear transects to explore the plume’s limits.6
Figure 7.5 Systematic–judgmental sampling plan for monitoring the leaching of pollutants from a landfill. The sampling sites, shown as red dots (•), are on a systematic grid straddling the direction of the groundwater’s flow. Sampling along linear transects helps establish the plume’s limits.
Stratified Sampling
Another combination of the three primary approaches to sampling is judgmental–random, or stratified sampling. Many target populations consist of distinct units, or strata. For example, suppose we are studying particulate Pb in urban air. Because particulates come in a range of sizes—some visible and some microscopic—and from many sources—road dust, diesel soot, and fly ash to name a few—we can subdivide the target population by size or source. If we choose a random sampling plan, then we collect samples without considering the different strata. For a stratified sampling, we divide the target population into strata and collect random samples from within each stratum. After analyzing the samples from each stratum, we pool their respective means to give an overall mean for the target population. The advantage of stratified sampling is that individual strata usually are more homogeneous than the target population. The overall sampling variance for stratified sampling is always at least as good, and often better than that obtained by simple random sampling.
Convenience Sampling
One additional method of sampling deserves brief mention. In convenience sampling we select sample sites using criteria other than minimizing sampling error and sampling variance. In a survey of rural groundwater quality, for example, we can choose to drill wells at randomly selected sites or we can make use of existing wells, which is usually the preferred choice. In this case cost, expedience, and accessibility are more important than ensuring a random sample.
7.2.2 What Type of Sample to Collect
After determining where to collect samples, the next step in designing a sampling plan is to decide what type of sample to collect. There are three common methods for obtaining samples: grab sampling, composite sampling, and in situ sampling.
The most common type of sample is a grab sample, in which we collect a portion of the target population at a specific time and/or location, providing a “snapshot” of the target population. If our target population is homogeneous, a series of random grab samples allows us to establish its properties. For a heterogeneous target population, systematic grab sampling allows us to characterize how its properties change over time and/or space.
A composite sample is a set of grab samples that we combine into a single sample before analysis. Because information is lost when we combine individual samples, we normally analyze grab sample separately. In some situations, however, there are advantages to working with a composite sample.
One situation where composite sampling is appropriate is when our interest is in the target population’s average composition over time or space. For example, wastewater treatment plants must monitor and report the average daily composition of the treated water they release to the environment. The analyst can collect and analyze individual grab samples using a systematic sampling plan, reporting the average result, or she can combine the grab samples into a single composite sample. Analyzing a single composite sample instead of many individual grab samples, saves time and money.
Composite sampling is also useful when a single sample can not supply sufficient material for the analysis. For example, analytical methods for determining PCB’s in fish often require as much as 50 g of tissue, an amount that may be difficult to obtain from a single fish. By combining and homogenizing tissue samples from several fish, it is easy to obtain the necessary 50-g sample.
A significant disadvantage of grab samples and composite samples is that we cannot use them to continuously monitor a time-dependent change in the target population. In situ sampling, in which we insert an analytical sensor into the target population, allows us to continuously monitor the target population without removing individual grab samples. For example, we can monitor the pH of a solution moving through an industrial production line by immersing a pH electrode in the solution’s flow.
Example 7.4
A study of the possible relationship between traffic density and the concentrations of lead, cadmium, and zinc in roadside soils, made use of the following sampling plan.7 Samples of surface soil (0–10 cm) were collected at perpendicular distances of 1, 5, 10, 20, and 30 m from the roadway. At each distance, 10 samples were taken from different locations and mixed to form a single sample. What type of sampling plan is this? Explain why this is an appropriate sampling plan.
Solution
This is an example of a systematic–judgemental sampling plan using composite samples. These are good choices given the goals of the study. Automobile emissions release particulates containing elevated concentrations of lead, cadmium, and zinc—this study was conducted in Uganda where leaded gasoline is still in use—which settle out on the surrounding roadside soils as “dry rain.” Sampling in areas near roadways, and sampling at fixed distances from the roadway provides sufficient data for the study, while limiting the total number of samples. Combining samples from the same distance into a single, composite sample has the advantage of decreasing sampling uncertainty. Because variations in metal concentrations perpendicular to the roadway is not of interest, the composite samples do not result in a loss of information.
7.2.3 How Much Sample to Collect
To minimize sampling errors, samples must be of an appropriate size. If a sample is too small, its composition may differ substantially from that of the target population, introducing a sampling error. Samples that are too large, however, require more time and money to collect and analyze, without providing a significant improvement in the sampling error.
Let’s assume that our target population is a homogeneous mixture of two types of particles. Particles of type A contain a fixed concentration of analyte, and particles without analyte are of type B. Samples from this target population follow a binomial distribution. (For a review of the binomial distribution, see Chapter 4.) If we collect a sample containing n particles, the expected number of particles containing analyte, nA, is
\[n_\textrm A=np\]
where p is the probability of selecting a particle of type A. The standard deviation for sampling is
\[s_\textrm{samp}=\sqrt{np(1-p)}\tag{7.3}\]
To calculate the relative standard deviation for sampling, ssamprel, we divide equation 7.3 by nA, obtaining
\[s_\textrm{samp}^\textrm{rel}=\dfrac{\sqrt{np(1-p)}}{np}\]
Solving for n allows us to calculate the number of particles providing the desired relative sampling variance.
\[n=\dfrac{1-p}{p}\times\dfrac{1}{(s_\textrm{samp}^\textrm{rel})^2}\tag{7.4}\]
Example 7.5
Suppose we are analyzing a soil where the particles containing analyte represent only 1 × 10–7% of the population. How many particles must we collect to give a percent relative standard deviation for sampling of 1%?
Solution
Since the particles of interest account for 1 × 10–7% of all particles, the probability, p, of selecting one of these particles is only 1 × 10–9. Substituting into equation 7.4 gives
\[n=\dfrac{1 - (1\times10^{-9})}{1\times10^{-9}}\times\dfrac{1}{(0.01)^2}=1\times10^{13}\]
To obtain a relative standard deviation for sampling of 1%, we need to collect 1 × 1013 particles.
A sample containing 1013 particles may be fairly large. Suppose this is equivalent to a mass of 80 g. Working with a sample this large clearly is not practical. Does this mean we must work with a smaller sample and accept a larger relative standard deviation for sampling? Fortunately the answer is no. An important feature of equation 7.4 is that the relative standard deviation for sampling is a function of the number of particles, not the combined mass of the particles. If we crush and grind the particles to make them smaller, then a sample containing 1013 particles will have a smaller mass. If we assume that a particle is spherical, then its mass is proportional to the cube of its radius.
\[\textrm{mass}\propto r^3\]
Decreasing a particle’s radius by a factor of 2, for example, decreases its mass by a factor of 23, or 8.
Example 7.6
Assume that a sample of 1013 particles from Example 7.5 weighs 80 g and that the particles are spherical. By how much must we reduce a particle’s radius if we wish to work with 0.6-g samples?
Solution
To reduce the sample’s mass from 80 g to 0.6 g, we must change its mass by a factor of
\[\dfrac{80}{0.6}=133\times\]
To accomplish this we must decrease a particle’s radius by a factor of
\[r^3=133\times\]
\[r=5.1\times\]
Decreasing the radius by a factor of approximately 5 allows us to decrease the sample’s mass from 80 g to 0.6 g.
Treating a population as though it contains only two types of particles is a useful exercise because it shows us that we can improve the relative standard deviation for sampling by collecting more particles. Of course, a real population contains more than two types of particles, with the analyte present at several levels of concentration. Nevertheless, many well-mixed populations, in which the population’s composition is homogeneous on the scale at which we sample, approximate binomial sampling statistics. Under these conditions the following relationship between the mass of a random grab sample, m, and the percent relative standard deviation for sampling, R, is often valid
\[mR^2=K_\textrm s\tag{7.5}\]
where Ks is a sampling constant equal to the mass of a sample producing a percent relative standard deviation for sampling of ±1%.8
Note
Problem 8 in the end of chapter problems asks you to derive equation 7.5.
Example 7.7
The following data were obtained in a preliminary determination of the amount of inorganic ash in a breakfast cereal.
| Mass of Cereal (g) | 0.9956 | 0.9981 | 1.0036 | 0.9994 | 1.0067 |
| % w/w Ash | 1.34 | 1.29 | 1.32 | 1.26 | 1.28 |
What is the value of Ks and what size samples are needed to give a percent relative standard deviation for sampling of ±2.0%. Predict the percent relative standard deviation and the absolute standard deviation if we collect 5.00-g samples.
Solution
To determine the sampling constant, Ks, we need to know the average mass of the cereal samples and the relative standard deviation for the amount of ash. The average mass of the cereal samples is 1.0007 g. The average % w/w ash and its absolute standard deviation are, respectively, 1.298% w/w and 0.03194% w/w. The percent relative standard deviation, R, therefore, is
\[R=\dfrac{s_\textrm{samp}}{\bar X}\times100=\mathrm{\dfrac{0.03194\%\;w/w}{1.298\%\;w/w}}\times100=2.46\%\]
Solving for Ks gives its value as
\[K_\textrm s=mR^2=\mathrm{(1.0007\;g)(2.46)^2=6.06\;g}\]
To obtain a percent relative standard deviation of ±2%, samples need to have a mass of at least
\[m=\dfrac{K_\textrm s}{R^2}=\mathrm{\dfrac{6.06\;g}{(2.0)^2}=1.5\;g}\]
If we use 5.00-g samples, then the expected percent relative standard deviation is
\[R=\sqrt{\dfrac{K_\textrm s}{m}}=\mathrm{\sqrt{\dfrac{6.06\;g}{5.00\;g}}=1.10\%}\]
and the expected absolute standard deviation is
\[s_\textrm{samp}=\dfrac{R\bar X}{100}=\mathrm{\dfrac{(1.10)(1.298\%\;w/w)}{100}=0.0143\%\;w/w}\]
Practice Exercise 7.3
Olaquindox is a synthetic growth promoter in medicated feeds for pigs. In an analysis of a production lot of feed, five samples with nominal masses of 0.95 g were collected and analyzed, with the results shown in the following table.
| mass (g) | 0.9530 | 0.9728 | 0.9660 | 0.9402 | 0.9576 |
| mg olaquindox/kg feed | 23.0 | 23.8 | 21.0 | 26.5 | 21.4 |
What is the value of Ks and what size samples are needed to obtain a percent relative deviation for sampling of 5.0%? By how much do you need to reduce the average particle size if samples must weigh no more than 1 g?
Click here to review your answer to this exercise.
7.2.4 How Many Samples to Collect
In the previous section we considered how much sample we need to minimize the standard deviation due to sampling. Another important consideration is the number of samples to collect. If our samples are normally distributed, then the confidence interval for the sampling error is
\[\mu=\bar X\pm\dfrac{ts_\textrm{samp}}{\sqrt{n_\textrm{samp}}}\tag{7.6}\]
where nsamp is the number of samples and ssamp is the standard deviation for sampling. Rearranging equation 7.6 and substituting e for the quantity X − μ, gives the number of samples as
\[n_\textrm{samp}=\dfrac{t^2s_\textrm{samp}^2}{e^2}\tag{7.7}\]
Because the value of t depends on nsamp, the solution to equation 7.7 is found iteratively.
Note
When we use equation 7.7, the standard deviation for sampling, ssamp, and the error, e, must be expressed in the same way. Because ssamp is given as a percent relative standard deviation, the error, e, is given as a percent relative error. When you use equation 7.7, be sure to check that you are expressing ssamp and e in the same way.
Example 7.8
In Example 7.7 we determined that we need 1.5-g samples to establish an ssamp of ±2.0% for the amount of inorganic ash in cereal. How many 1.5-g samples do we need to obtain a percent relative sampling error of ±0.80% at the 95% confidence level?
Solution
Because the value of t depends on the number of samples—a result we have yet to calculate—we begin by letting nsamp = ∞ and using t(0.05, ∞) for t. From Appendix 4, the value for t(0.05, ‡) is 1.960. Substituting known values into equation 7.7 gives the number of samples as
\[n_\textrm{samp}=\dfrac{(1.960)^2(2.0)^2}{(0.80)^2}=24.0\approx24\]
Letting nsamp = 24, the value of t(0.05, 23) from Appendix 4 is 2.073. (With 24 samples, the degrees of freedom for t is 23.) Recalculating nsamp gives
\[n_\textrm{samp}=\dfrac{(2.073)^2(2.0)^2}{(0.80)^2}=26.9\approx27\]
When nsamp = 27, the value of t(0.05, 26) from Appendix 4 is 2.060. Recalculating nsamp gives
\[n_\textrm{samp}=\dfrac{(2.060)^2(2.0)^2}{(0.80)^2}=26.52\approx27\]
Because two successive calculations give the same value for nsamp, we have an iterative solution to the problem. We need 27 samples to achieve a percent relative sampling error of ±0.80% at the 95% confidence level.
Practice Exercise 7.4
Assuming that the percent relative standard deviation for sampling in the determination of olaquindox in medicated feed is 5.0% (see Practice Exercise 7.3), how many samples do we need to analyze to obtain a percent relative sampling error of ±2.5% at α = 0.05?
Click here to review your answer to this exercise.
Equation 7.7 provides an estimate for the smallest number of samples that will produce the desired sampling error. The actual sampling error may be substantially larger if ssamp for the samples we collect during the subsequent analysis is greater than ssamp used to calculate nsamp. This is not an uncommon problem. For a target population with a relative sampling variance of 50 and a desired relative sampling error of ±5%, equation 7.7 predicts that 10 samples are sufficient. In a simulation using 1000 samples of size 10, however, only 57% of the trials resulted in a sampling error of less than ±5%.9 Increasing the number of samples to 17 was sufficient to ensure that the desired sampling error was achieved 95% of the time.
Note
For an interesting discussion of why the number of samples is important, see Kaplan, D.; Lacetera, N.; Kaplan, C. “Sample Size and Precision in NIH Peer Review,” Plos One, 2008, 3(7), 1–3. When reviewing grants, individual reviewers report a score between 1.0 and 5.0 (two significant figure). NIH reports the average score to three significant figures, implying that differences of 0.01 are significant. If the individual scores have a standard deviation of 0.1, then a difference of 0.01 is significant at α = 0.05 only if there are 384 reviews. The authors conclude that NIH review panels are too small to provide a statistically meaningful separation between proposals receiving similar scores.
7.2.5 Minimizing the Overall Variance
A final consideration when developing a sampling plan is to minimize the overall variance for the analysis. Equation 7.2 shows that the overall variance is a function of the variance due to the method, smeth2, and the variance due to sampling, ssamp2. As we have seen, we can improve the sampling variance by collecting more samples of the proper size. Increasing the number of times we analyze each sample improves the method’s variance. If ssamp2 is significantly greater than smeth2, we can ignore the method’s contribution to the overall variance and use equation 7.7 to estimate the number of samples to analyze. Analyzing any sample more than once will not improve the overall variance, because the method’s variance is insignificant.
If smeth2 is significantly greater than ssamp2, then we need to collect and analyze only one sample. The number of replicate analyses, nrep, needed to minimize the error due to the method is given by an equation similar to equation 7.7.
\[n_\textrm{rep}=\dfrac{t^2s_\textrm{meth}^2}{e^2}\]
Unfortunately, the simple situations described above are often the exception. For many analyses, both the sampling variance and the method variance are significant, and both multiple samples and replicate analyses of each sample are necessary. The overall error in this case is
\[e=t\sqrt{\dfrac{s_\textrm{samp}^2}{n_\textrm{samp}}+\dfrac{s_\textrm{meth}^2}{n_\textrm{samp}n_\textrm{rep}}}\tag{7.8}\]
Equation 7.8 does not have a unique solution as different combinations of nsamp and nrep give the same overall error. How many samples we collect and how many times we analyze each sample is determined by other concerns, such as the cost of collecting and analyzing samples, and the amount of available sample.
Example 7.9
An analytical method has a percent relative sampling variance of 0.40% and a percent relative method variance of 0.070%. Evaluate the percent relative error (α = 0.05) if you collect 5 samples, analyzing each twice, and if you collect 2 samples, analyzing each 5 times.
Solution
Both sampling strategies require a total of 10 analyses. From Appendix 4 we find that the value of t(0.05, 9) is 2.262. Using equation 7.8, the relative error for the first sampling strategy is
\[e=2.262\sqrt{\dfrac{0.40}{5}+\dfrac{0.070}{5\times2}}=0.67\%\]
and that for the second sampling strategy is
\[e=2.262\sqrt{\dfrac{0.40}{2}+\dfrac{0.070}{2\times5}}=1.0\%\]
Because the method variance is smaller than the sampling variance, we obtain a smaller relative error if we collect more samples, analyzing each fewer times.
Practice Exercise 7.5
An analytical method has a percent relative sampling variance of 0.10% and a percent relative method variance of 0.20%. The cost of collecting a sample is $20 and the cost of analyzing a sample is $50. Propose a sampling strategy that provides a maximum percent relative error of ±0.50% (α = 0.05) and a maximum cost of $700.
Click here to review your answer to this exercise.