13.1: Kinetic Methods Versus Equilibrium Methods
- Page ID
- 5586
In an equilibrium method the analytical signal is determined by an equilibrium reaction involving the analyte or by a steady-state process that maintains the analyte’s concentration. When we determine the concentration of iron in water by measuring the absorbance of the orange-red Fe(phen)32+ complex (see Representative Method 10.1), the signal depends upon the concentration of Fe(phen)32+, which, in turn, is determined by the complex’s formation constant. In the flame atomic absorption determination of Cu and Zn in tissue samples (see Representative Method 10.2), the concentration of each metal in the flame remains constant because each step in the process of atomizing the sample is in a steady-state. In a kinetic method the analytical signal is determined by the rate of a reaction involving the analyte, or by a nonsteady-state process. As a result, the analyte’s concentration changes during the time in which we are monitoring the signal.
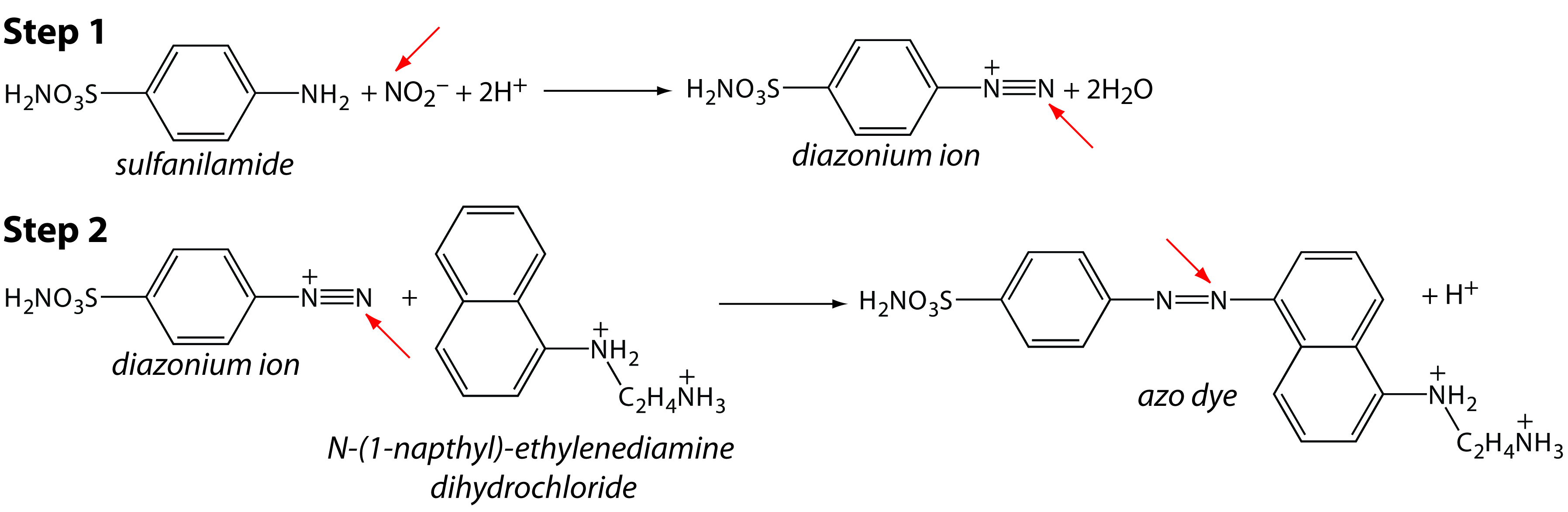
In many cases, we can complete an analysis using either an equilibrium method or a kinetic method by simply changing the time at which we choose to measure the analytical signal. For example, one method for determining the concentration of nitrite, NO2–, in groundwater utilizes the two-step diazotization reaction shown in Figure 13.1.1 The final product, which is a reddish-purple azo dye, absorbs visible light at a wavelength of 543 nm. Because neither reaction in Figure 13.1 is rapid, the absorbance—which is directly proportional to the concentration of NO2–—is measured 10 min after adding the last reagent, ensuring that it reaches the steady-state value required of an equilibrium method.
Figure 13.1 Analytical scheme for the analysis of NO2– in groundwater. The red arrow highlights the nitrogen in NO2– that becomes part of the azo dye.
We can use the same set of reactions as the basis for a kinetic method by measuring the absorbance during the 10-min development period, obtaining information about the reaction’s rate. If the rate is a function of the concentration of NO2–, then we can use the rate to determine its concentration in the sample.2
There are many potential advantages to kinetic methods of analysis, perhaps the most important of which is the ability to use chemical reactions and systems that are slow to reach equilibrium. In this chapter we examine three techniques that rely on measurements made while the analytical system is under kinetic control: chemical kinetic techniques, in which we measure the rate of a chemical reaction; radiochemical techniques, in which we measure the decay of a radioactive element; and flow injection analysis, in which we inject the analyte into a continuously flowing carrier stream, where its mixing with and reaction with reagents in the stream are controlled by the kinetic processes of convection and diffusion.