16.6: Batteries and Fuel Cells
- Page ID
- 264
Make sure you thoroughly understand the following essential ideas which have been presented below.
- A battery is a galvanic cell in which some of the free energy change associated with a spontaneous electron-transfer reaction is captured in the form of electrical energy.
- A secondary or storage battery is one in which the electron-transfer reaction can be reversed by applying a charging current from an external source.
- A fuel cell is a special type of battery in which the reactants are supplied from an external source as power is produced. In most practical fuel cells, H+ions are produced at the anode (either from H2 or a hydrocarbon) and oxygen from the air is reduced to H2O at the cathode.
- The cathodic reduction of O2 is kinetically limited, necessitating the use of electrode surfaces having high catalytic activity.
- The electrodes in batteries must have very high effective surface areas, and thus be highly porous. This requirement may conflict with the other important one of efficient diffusion of reactants and products in the narrow channels within the pores.
- Batteries and fuel cells designed to power vehicles and portable devices need to have high charge-to-weight and charge-tovolume ratios.
One of the oldest and most important applications of electrochemistry is to the storage and conversion of energy. You already know that a galvanic cell converts chemical energy to work; similarly, an electrolytic cell converts electrical work into chemical free energy. Devices that carry out these conversions are called batteries. In ordinary batteries the chemical components are contained within the device itself. If the reactants are supplied from an external source as they are consumed, the device is called a fuel cell.
Introduction
The term battery derives from the older use of this word to describe physical attack or "beating"; Benjamin Franklin first applied the term to the electrical shocks that could be produced by an array of charged glass plates. In common usage, the term "call" is often used in place of battery. For portable and transportation applications especially, a battery or fuel cell should store (and be able to deliver) the maximum amount of energy at the desired rate (power level) from a device that has the smallest possible weight and volume. The following parameters are commonly used to express these attributes:
- Storage capacity or charge density, coulombs/liter or coulombs/kg;
- Energy density, J/kg or watt-hour/lb
- Power density, watts/kg
- Voltage efficiency, ratio of output voltage to E°
- Lifetime: shelf-life (resistance to self-discharge) or charge/recharge cycles
Primary and Secondary Batteries
A secondary or storage battery is capable of being recharged; its electrode reactions can proceed in either direction. During charging, electrical work is done on the cell to provide the free energy needed to force the reaction in the non-spontaneous direction. A primary cell, as expemplified by an ordinary flashlight battery, cannot be recharged with any efficiency, so the amount of energy it can deliver is limited to that obtainable from the reactants that were placed in it at the time of manufacture.
The lead-acid storage cell
The most well-known storage cell is the lead-acid cell, which was invented by Gaston Planté in 1859 and is still the most widely used device of its type. The cell is represented by
\[Pb(s) | PbSO_4(s) | H_2SO_4(aq) || PbSO_4(s), PbO_2(s) | Pb(s)\]
and the net cell reaction is
\[Pb(s) + PbO_2(s) + 2 H_2SO_4(aq) → 2 PbSO_4(s) + 2 H_2O\]
The reaction proceeds to the right during discharge and to the left during charging. The state of charge can be estimated by measuring the density of the electrolyte; sulfuric acid is about twice as dense as water, so as the cell is discharged, the density of the electrolyte decreases.
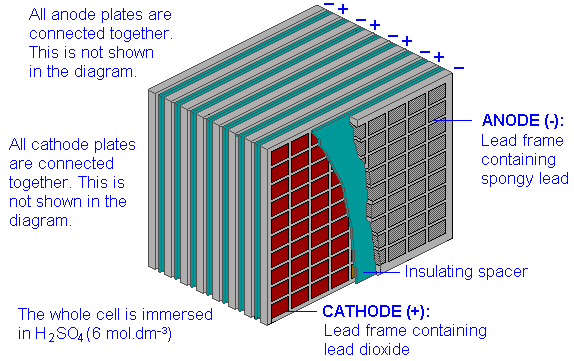
The technology of lead-acid storage batteries has undergone remarkably little change since the late 19th century. Their main drawback as power sources for electric vehicles is the weight of the lead; the maximum energy density is only about 35 Ah/kg, and actual values may be only half as much. There are also a few other problems:
- The sulfuric acid electrolyte becomes quite viscous when the temperature is low, inhibiting the flow of ions between the plates and reducing the current that can be delivered. This effect is well-known to anyone who has had difficulty starting a car in cold weather.
- These batteries tend to slowly self-discharge, so a car left idle for several weeks might be unable to start.
- Over time, PbSO4 that does not get converted to PbO2 due to lack of complete discharge gradually changes to an inert form which limits the battery capacity. Also, "fast" charging causes rapid evolution of hydrogen from the water in the electrolyte; the bubbles form on the lead surface and can tear PbO2 off the positive plate. Eventually enough solid material accumulates at the bottom of the electrolyte to short-circuit the battery, leading to its permanent demise.
The LeClanché "dry cell"
The most well-known primary battery has long been the common "dry cell" that is widely used to power flashlights and similar devices. The modern dry cell is based on the one invented by Georges Leclanché in 1866. The electrode reactions are
\[Zn → Zn^{2+} + 2e^–\]
\[2 MnO_2 + 2H^+ + 2e^– → Mn_2O_3 + H_2O\]
Despite its name, this cell is not really "dry"; the electrolyte is a wet paste containing NH4Cl to supply the hydrogen ions. The chemistry of this cell is more complicated than it would appear from these equations, and there are many side reactions and these cells have limited shelf-lifes due to self discharge. (In some of the older ones, attack by the acidic ammonium ion on the zinc would release hydrogen gas, causing the battery to swell and rupture, often ruining an unused flashlight or other device.) A more modern version, introduced in 1949, is the alkaline cell which employs a KOH electrolyte and a zinc-powder anode which permits the cell to deliver higher currents and avoids the corrosive effects of the acidic ammonium ion on the zinc.
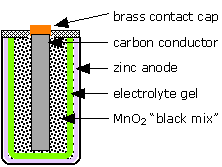
What is the cell notation for the LeClanché dry cell?
Physical limitations of Battery Performance
The most important of these are:
- Effective surface area of the electrode. A 1-cm2 sheet of polished metal presents far less active surface than does one that contains numerous surface projections or pores. All useful batteries and fuel cells employ highly porous electrodes. Recent advances in nanotechnology are likely to greatly improve this parameter.
- Current density of electrode surface. Expressed in amperes m–2, this is essentially a measure of the catalytic ability of the electrode, that is, its ability to reduce the activation energy of the electron transfer process.
- Rate at which electroactive components can be delivered to or depart from the active electrode surface. These processes are controlled by thermal diffusion and are inhibited by the very narrow pores that are needed to produce the large active surface area.
- Side reactions and irreversible processes. The products of the discharge reaction may tend to react with the charge-storing components. Thermal diffusion can also cause self-discharge, limiting the shelf life of the battery. Recharging of some storage batteries may lead to formation of less active modifications of solid phases, thus reducing the number of charge/discharge cycles possible.
Clearly, these are all primarily kinetic and mechanistic factors which require a great deal of experimentation to understand and optimize.
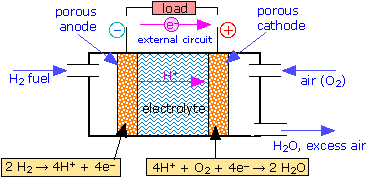
The Fuel Cell
Conventional batteries supply electrical energy from the chemical reactants stored within them; when these reactants are consumed, the battery is "dead". An alternative approach would be to feed the reactants into the cell as they are required, so as to permit the cell to operate continuously. In this case the reactants can be thought of as "fuel" to drive the cell, hence the term fuel cell.
Although fuel cells were not employed for practical purposes until space exploration began in the 1960's, the principle was first demonstrated in 1839 by Sir William Grove, a Welsh lawyer and amateur chemist. At the time, it was already known that water could be decomposed into hydrogen and oxygen by electrolysis; Grove tried recombining the two gases in a simple apparatus, and discovered what he called "reverse electrolysis"— that is, the recombination of H2 and O2 into water— causing a potential difference to be generated between the two electrodes:
| H2(g) → 2 H+ + 2e– | E° = 0 v | |
| ½ O2 + 2 H+ + 2e– → H2O(l) | E° = +1.23 v | |
| H2(g) + ½ O2(g) → H2O(l) | E° = +1.23 v |
It was not until 1959 that the first working hydrogen-oxygen fuel cell was developed by Francis Thomas Bacon in England. Modern cells employ an alkaline electrolyte, so the electrode reactions differ from the one shown above by the addition of OH– to both sides of the equations (note that the net reaction is the same):
| H2(g) + 2 OH– → 2 H2O + 2 e– | E° = 0 v | |
| ½ O2 (g) + 2 H2O + 2 e– → 2 OH– | E° = +1.23 v | |
| H2(g) + ½ O2(g) → H2O | E° = +1.23 v |
Although hydrogen has the largest energy-to-mass ratio of any fuel, it cannot be compressed to a liquid at ordinary temperatures. If it is stored as a gas, the very high pressures require heavy storage containers, greatly reducing its effective energy density. Some solid materials capable of absorbing large amount of H2 can reduce the required pressure. Other fuels such as alcohols, hydrocarbon liquids, and even coal slurries have been used; methanol appears to be an especially promising fuel.
One reason for the interest in fuel cells is that they offer a far more efficient way of utilizing chemical energy than does conventional thermal conversion. The work obtainable in the limit of reversible operation of a fuel cell is 229 kJ per mole of H2O formed. If the hydrogen were simply burned in oxygen, the heat obtainable would be ΔH = 242 kJ mol–1, but no more than about half of this heat can be converted into work so the output would not exceed 121 kJ mol–1. This limit is a consequence of the Second Law of Thermodynamics. The fraction of heat that can be converted into work (\(\eta\)) is a function of how far (in temperature) the heat falls as it flows through the engine and into the surroundings; this fraction is given by
\[\eta=\dfrac{1 - T_{high}}{T_{low}}\]
At normal environmental temperatures of around 300 K, this would have to be at least 600 K for 50% thermal efficiency.
The major limitation of present fuel cells is that the rates of the electrode reactions, especially the one in which oxygen is reduced, tend to be very small, and thus so is the output current per unit of electrode surface. Coating the electrode with a suitable catalytic material is almost always necessary to obtain usable output currents, but good catalysts are mostly very expensive substances such as platinum, so that the resulting cells are too costly for most practical uses. There is no doubt that if an efficient, low-cost catalytic electrode surface is ever developed, the fuel cell would become a mainstay of the energy economy.
Microbobial Fuel Cells
Certain types of bacteria are able to oxidize organic compounds to carbon dioxide while directly transferring electrons to electrodes. These so-called electricigen organisms may make it possible to convert renewable biomass and organic waste directly into electricity without the wasted energy and pollution produced by direct combustion. In one experiment, a graphite electrode immersed in ordinary mud (containing humic materials) was able to produce measurable amounts of electricity.
Summary
Make sure you thoroughly understand the following essential ideas which have been presented above. It is especially imortant that you know the precise meanings of all the highlighted terms in the context of this topic.
- A battery is a galvanic cell in which some of the free energy change associated with a spontaneous electron-transfer reaction is captured in the form of electrical energy.
- A secondary or storage battery is one in which the electron-transfer reaction can be reversed by applying a charging current from an external source.
- A fuel cell is a special type of battery in which the reactants are supplied from an external source as power is produced. In most practical fuel cells, H+ ions are produced at the anode (either from H2 or a hydrocarbon) and oxygen from the air is reduced to H2O at the cathode.
- The cathodic reduction of O2 is kinetically limited, necessitating the use of electrode surfaces having high catalytic activity.
- The electrodes in batteries must have very high effective surface areas, and thus be highly porous. This requirement may conflict with the other important one of efficient diffusion of reactants and products in the narrow channels within the pores.
- Batteries and fuel cells designed to power vehicles and portable devices need to have high charge-to-weight and charge-tovolume ratios.


