Fragmentation
- Page ID
- 4185
When molecules go through a mass spectrometer, some of them arrive intact at the detector, but many of them break into pieces in a variety of different ways. To establish a charge on a molecule, an electron had to be removed; removal of that electron is effected through a collision, usually with a high-energy electron. During that collision, energy is transferred from the high-energy electron to the molecule, and that energy has to go somewhere. Part of it gets partitioned into various bond vibrations, so bonds start to vibrate quite a lot, until some of them snap completely. The molecular ion breaks apart and forms a fragment ion.
Some fragment ions are very common in mass spectrometry. These ions are seen frequently for either of two reasons:
- there is not a pathway available to break the ion down.
- the ion is relatively stable, so it forms easily.
Fragmentations occur through well-defined pathways or mechanisms. A mechanism is a step-by-step series of events that happens in a reaction. It is important to understand how reactions happen, but we will look at fragmentations when we study radical reactions.
However, it is useful to know what factors make cations stable.
Some Common Ions
There are a number of ions commonly seen in mass spectrometry that tell you a little bit about the structure. Just like with anions, there are a couple of common factors influence cation stability:
- Electronegativity plays a role. More electronegative atoms are less likely to be cations.
- Polarizability also plays a role. More polarizable atoms are more likely to be cations.
However, in most cases, we will be looking at carbon with a positive charge, and there are additional factors to distinguish between them
- Delocalization stabilizes a cation by spreading out the charge onto two or more different atoms.
- In Lewis structure terms, the easiest way to delocalize charge is via resonance.
- Resonance can involve other carbons, like in allyl and benzyl cations.
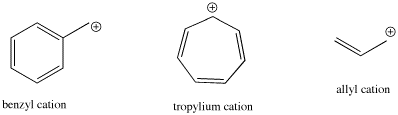
- Resonance can also involve other atoms, like in acylium or iminium cations.

- Delocalization can also be accomplished through inductive effects. The trend in carbocations is that the more substituents on teh carbocation, the greater the stability.
- Tertiary cations, with three substituents on the carbocation, are more stable than secondary cations, with two substituents on the carbocation. Secondary cations are more stable than primary ones. Primary cations are more stable than methyl cations.
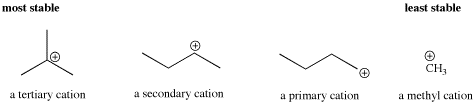
Molecular orbital calculations suggest that the cation is stabilized through interaction with neighboring C-H bonds in the alkyl groups. Specifically, a C-H sigma bonding orbital has symmetry similar to the empty p orbital on the positive carbon. The lobes on the two orbitals can overlap such that they are in phase, and that allows electrons to be donated from the C-H bond to the central, electron-deficient carbon. Formally, there is a bonding interaction and an antibonding interaction between these two orbitals. Since one of these orbitals is empty, the antibonding combination remains unoccupied. The bonding combination is populated, however, and since it is lower in energy than either the p orbital or the C-H sigma bond (all bonding combinations are lower in energy than the orbitals that combine to form them), there is a net decrease in energy.
Problem MS8.
Draw as many resonance structures as you can that help explain teh stability of the following cations:
a) allyl cation b) benzyl cation c) tropylium cation d) an acylium ion e) an iminium ion


