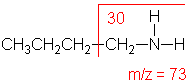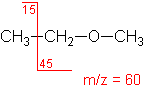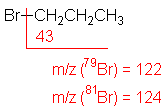Mass Spectrometry - Fragmentation Patterns
- Page ID
- 327
Following are examples of compounds listed by functional group, which demonstrate patterns which can be seen in mass spectra of compounds ionized by electron impact ionization. These examples do not provide information about the fragmentation mechanisms that cause these patterns. Additional information can be found in mass spectrometry reference books.
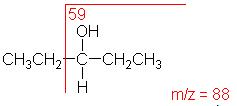
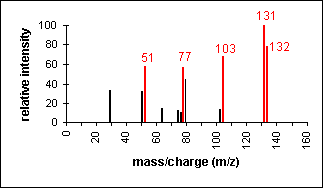
Alcohol
An alcohol's molecular ion is small or non-existent. Cleavage of the C-C bond next to the oxygen usually occurs. A loss of H2O may occur as in the spectra below.
3-Pentanol (C5H12O) with MW = 88.15
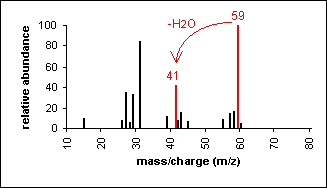 |
|
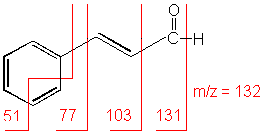
Aldehyde
Cleavage of bonds next to the carboxyl group results in the loss of hydrogen (molecular ion less 1) or the loss of CHO (molecular ion less 29).
3-Phenyl-2-propenal (C9H8O) with MW = 132.16
|
|
|
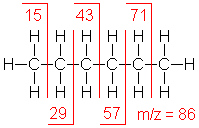
Alkane
Molecular ion peaks are present, possibly with low intensity. The fragmentation pattern contains clusters of peaks 14 mass units apart (which represent loss of (CH2)nCH3).
Hexane (C6H14) with MW = 86.18
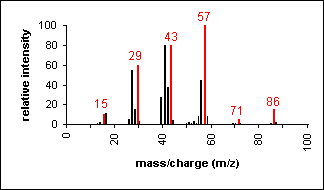 |
|
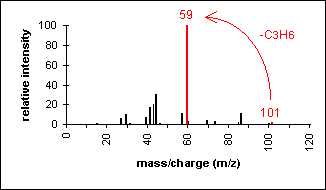
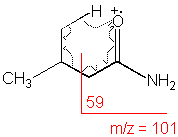
Amide
Primary amides show a base peak due to the McLafferty rearrangement.
3-Methylbutyramide (C5H11NO) with MW = 101.15
|
|
|
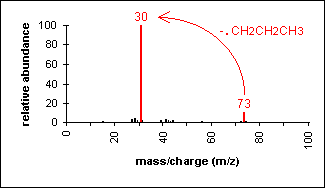
Amine
Molecular ion peak is an odd number. Alpha-cleavage dominates aliphatic amines.
n-Butylamine (C4H11N) with MW = 73.13
|
|
|
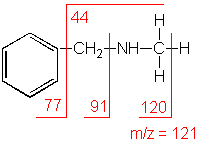
Another example is a secondary amine shown below. Again, the molecular ion peak is an odd number. The base peak is from the C-C cleavage adjacent to the C-N bond.
n-Methylbenzylamine (C8H11N) with MW = 121.18
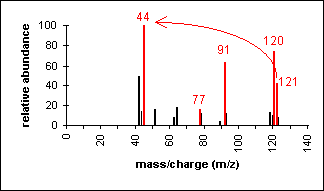 |
|
Aromatic
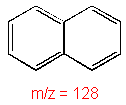
Molecular ion peaks are strong due to the stable structure.
Naphthalene (C10H8) with MW = 128.17
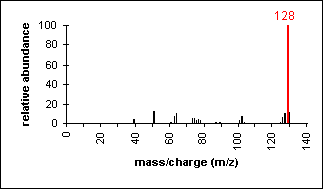 |
|
Carboxylic Acid
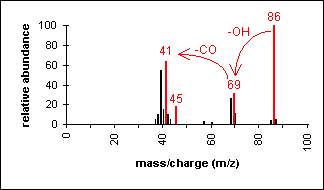
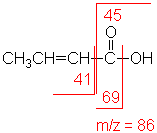
In short chain acids, peaks due to the loss of OH (molecular ion less 17) and COOH (molecular ion less 45) are prominent due to cleavage of bonds next to C=O.
2-Butenoic acid (C4H6O2) with MW = 86.09
|
|
|
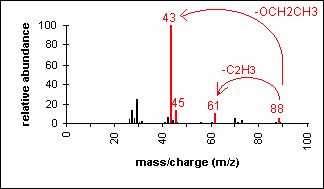
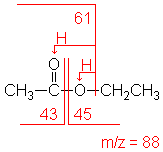
Ester
Fragments appear due to bond cleavage next to C=O (alkoxy group loss, -OR) and hydrogen rearrangements.
Ethyl acetate (C4H8O2) with MW = 88.11
|
|
|
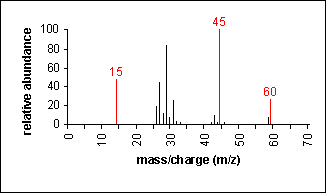
Ether
Fragmentation tends to occur alpha to the oxygen atom (C-C bond next to the oxygen).
Ethyl methyl ether (C3H8O) with MW = 60.10
|
|
|
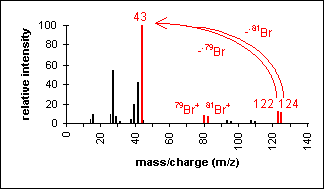
Halide
The presence of chlorine or bromine atoms is usually recognizable from isotopic peaks.
1-Bromopropane (C3H7Br) with MW = 123.00
|
|
|
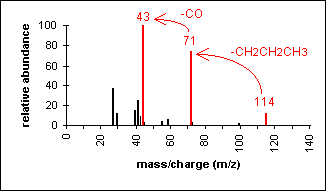
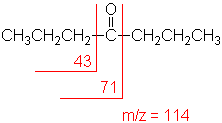
Ketone
Major fragmentation peaks result from cleavage of the C-C bonds adjacent to the carbonyl.
4-Heptanone (C7H14O) with MW = 114.19
|
|
|
Contributors and Attributions
Dr. Linda Breci, Associate Director Arizona Proteomics Consortium University of Arizona