ATP/ADP
- Page ID
- 376
Adenosine-5'-triphosphate (ATP) is comprised of an adenine ring, a ribose sugar, and three phosphate groups. ATP is often used for energy transfer in the cell. ATP synthase produces ATP from ADP or AMP + Pi. ATP has many uses. It is used as a coenzyme, in glycolysis, for example. ATP is also found in nucleic acids in the processes of DNA replication and transcription. In a neutral solution, ATP has negatively charged groups that allow it to chelate metals. Usually, Mg2+ stabilizes it.
Introduction
ATP is an unstable molecule which hydrolyzes to ADP and inorganic phosphate when it is in equilibrium with water. The high energy of this molecule comes from the two high-energy phosphate bonds. The bonds between phosphate molecules are called phosphoanhydride bonds. They are energy-rich and contain a ΔG of -30.5 kJ/mol.
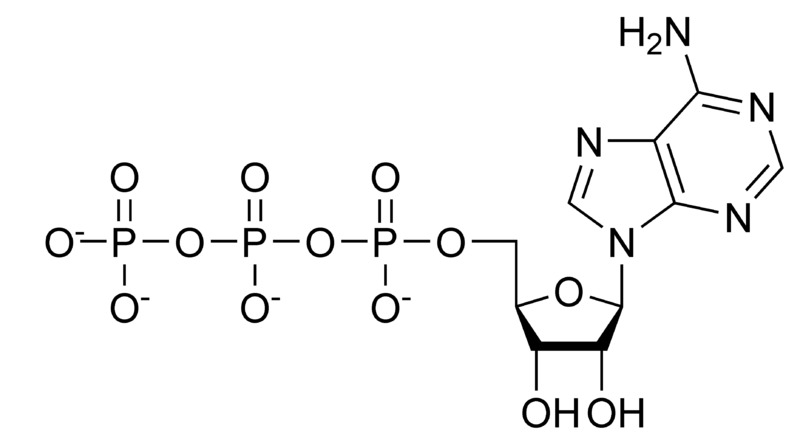
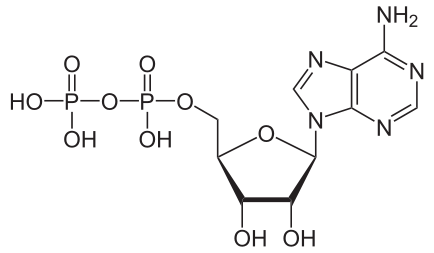
Hydrolysis of ATP
Removing or adding one phosphate group interconverts ATP to ADP or ADP to AMP. Breaking one phosphoanhydride bond releases 7.3 kcal/mol of energy.
\[\ce{ATP + H_2O \rightarrow ADP + P_{i}} \tag{ΔG = -30.5 kJ/mol}\]
\[\ce{ATP + H_2O \rightarrow AMP + 2 P_{i} } \tag{ΔG = -61 kJ/mol}\]
\[\ce{2 ADP + H_2O \rightarrow 2 AMP + 2 P_{i}} \tag{ΔG = -61 kJ/mol}\]
At pH 7,
\[\ce{ATP ^{4-} + H_2O \rightleftharpoons ADP^{3-} + HPO_4^{2-} + H^{+}} \nonumber\]
Why is ATP hydrolysis an exergonic reaction?
- The entropy, which is the level of disorder, of ADP is greater than that of ATP. Therefore, due to thermodynamics, the reaction spontaneously occurs because it wants to be at a higher entropy level. Also, the Gibbs' free energy of ATP is higher than that of ADP. Naturally, molecules want to be at a lower energy state, so equilibrium is shifted towards ADP.
- Electrostatic repulsion of the four negative charges on the oxygens of the ATP molecule. Naturally, like charges repel and opposite charges attract. Therefore, if there are four negative charges in close proximity to one another, they will naturally repel each other. This makes ATP a relatively unstable molecule because it will want to give away its phosphate groups, when given the chance, in order to become a more stable molecule.
- Resonance stabilization of ADP and of Pi is greater than that of ATP. The oxygen molecules of the ADP are sharing electrons. Those electrons are constantly being passed back and forth between the oxygens, creating an effect called resonance. This stables the ADP. Resonance does not occur in ATP; therefore, it is a more unstable molecule.
- There is a greater degree of solvation of Pi, H+, and ADP, relative to ATP. This means that it is easier for ATP to lose one of its phosphate groups. But, it takes a large amount of water to force ADP to lose one of its phosphates.
ATP in the Cell
ATP is the primary energy transporter for most energy-requiring reactions that occur in the cell. The continual synthesis of ATP and the immediate usage of it results in ATP having a very fast turnover rate. This means that ADP is synthesized into ATP very quickly and vice versa. For example, it takes only a few seconds for half of the ATP molecules in a cell to be converted into ADP to be used in driving endergonic (non-spontaneous) reactions and then converted back into ATP using exergonic (spontaneous) reactions.
ATP is useful in many cell processes such as glycolysis, photosynthesis, beta oxidation, anaerobic respiration, active transport across cell membranes (as in the electron transport chain), and synthesis of macromolecules such as DNA.
References
- Zubay, Geoffrey. Biochemistry. New York: Macmillan Publishing Company, 1988.
Problems
- In cellular respiration, which process produces the most ATP?
- True or false: ATP may be used to regulate certain enzymes.
- From one molecule of glucose, how many molecules of ATP will be produced?
- Where is ATP synthase located?
- True or false: ATP is generated through substrate level phosphorylation.
Answers
- Electron transport chain
- True
- 32 - 34 molecules of ATP
- It is located in the inner mitochondrial membrane.
- True
Contributors and Attributions
- Tiffany Lui, University of California, Davis.

