Enzyme Inhibition
- Page ID
- 416
Although activation of enzymes may be exploited therapeutically, most effects are produced by enzyme inhibition.
Direct Enzyme Inhibition
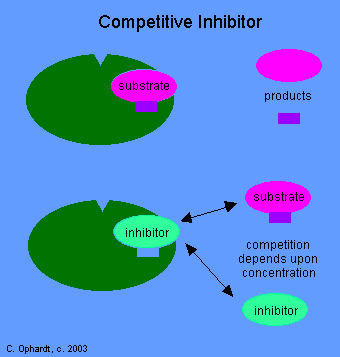
Inhibition caused by drugs may be either reversible or irreversible. A reversible situation occurs when an equilibrium can be established between the enzyme and the inhibitory drug. A competitive inhibition occurs when the drug, as "mimic" of the normal substrate competes with the normal substrate for the active site on the enzyme. Concentration effects are important for competitive inhibition.
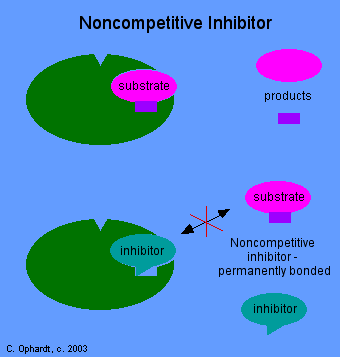
In noncompetitive inhibition, the drug combines with an enzyme, at a different site other than the active site. The normal substrate can not displace the drug from this site and can not interact with the active either since the shape of the enzyme has been altered. Among the many types of drugs that act as enzyme inhibitors the following may be included: antibiotics, acetylchlolinesterase agents, certain antidepressants such as monoamine oxidase inhibitors and some diuretics.
Suppression of Gene Function
Many drugs act as suppressors of gene function including antibiotics, fungicides, antimalarials and antivirals. Gene function may be suppressed in several steps of protein synthesis or inhibition of nucleic acid biosynthesis. Many substances which inhibit nucleic acid biosynthesis are very toxic since the drug is not very selective in its action between the parasite and host.
Antimetabolites
The strategy of chemotherapy consists of exploiting the biochemical differences between the host and parasite cells. Metabolites are any substances used or produced by biochemical reactions. A drug which possesses a remarkably close chemical similarity (mimic) to the normal metabolite is called an antimetabolite. The antimetabolite enters a normal synthetic reaction by "fooling" an enzyme and producing a counterfeit metabolite. The counterfeit metabolite inhibits another enzyme or is an unusable fraudulent end product which cannot be utilized by the cell for growth or reproduction. Such antimetabolites have been used as antibacterial or anticancer agents.
Contributors
- Charles Ophardt, Professor Emeritus, Elmhurst College; Virtual Chembook

