Nucleotides
- Page ID
- 469
Nucleotides are the basic monomer building block units in the nucleic acids. A nucleotide consists of a phosphate, pentose sugar, and a heterocyclic amine.
Adenosine 5'-monophosphate (AMP)
The phosphoric acid forms a phosphate-ester bond with the alcohol on carbon #5 in the pentose. A nitrogen in the heterocyclic amines displaces the -OH group on carbon #1 of the pentose. The reaction is shown in the graphic below. If the sugar is ribose, the general name is ribonucleotide and deoxyribonucleotide if the sugar is deoxyribose. The other four nucleotides are synthesized in a similar fashion.

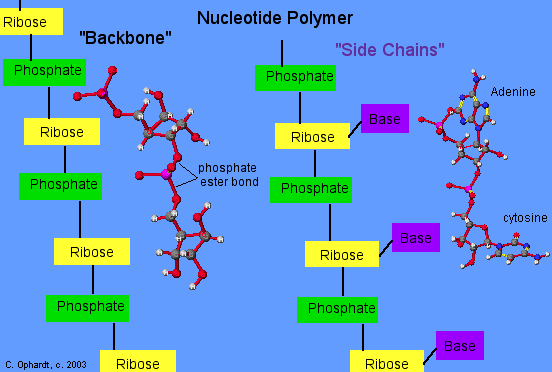
Polymeric Nucleotides
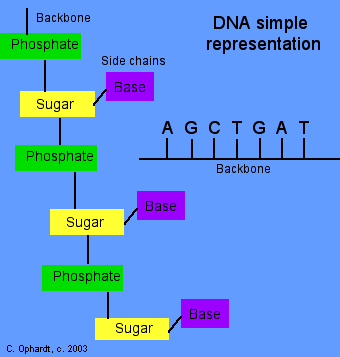
Simple Representation of Nucleotide Polymer
Just as the exact amino acid sequence is important in proteins, the sequence of heterocyclic amine bases determines the function of the DNA and RNA. This sequence of bases on DNA determines the genetic information carried in each cell. Currently, much research is under way to determine the heterocyclic amine sequences in a variety of RNA and DNA molecules. The Genome Project as already succeeded in determining the DNA sequences in humans and other organisms. Future research will be to determine the exact functions of each DNA segment as these contain the codes for protein synthesis.
Contributors
- Charles Ophardt, Professor Emeritus, Elmhurst College; Virtual Chembook

