Anti-Cancer Drugs I
- Page ID
- 410
The available anticancer drugs have distinct mechanisms of action which may vary in their effects on different types of normal and cancer cells. A single "cure" for cancer has proved elusive since there is not a single type of cancer but as many as 100 different types of cancer. In addition, there are very few demonstrable biochemical differences between cancerous cells and normal cells. For this reason the effectiveness of many anticancer drugs is limited by their toxicity to normal rapidly growing cells in the intestinal and bone marrow areas. A final problem is that cancerous cells which are initially suppressed by a specific drug may develop a resistance to that drug. For this reason cancer chemotherapy may consist of using several drugs in combination for varying lengths of time.
Introduction
Chemotherapy drugs, are sometimes feared because of a patient's concern about toxic effects. Their role is to slow and hopefully halt the growth and spread of a cancer. There are three goals associated with the use of the most commonly-used anticancer agents.
- Damage the DNA of the affected cancer cells.
- Inhibit the synthesis of new DNA strands to stop the cell from replicating, because the replication of the cell is what allows the tumor to grow.
- Stop mitosis or the actual splitting of the original cell into two new cells. Stopping mitosis stops cell division (replication) of the cancer and may ultimately halt the progression of the cancer.
Unfortunately, the majority of drugs currently on the market are not specific, which leads to the many common side effects associated with cancer chemotherapy. Because the common approach of all chemotherapy is to decrease the growth rate (cell division) of the cancer cells, the side effects are seen in bodily systems that naturally have a rapid turnover of cells iincluding skin, hair, gastrointestinal, and bone marrow. These healthy, normal cells, also end up damaged by the chemotherapy program.
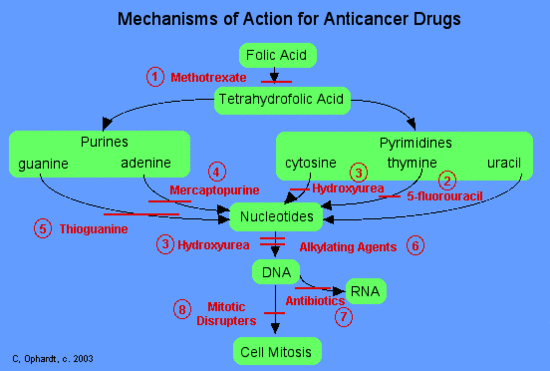
Categories of Chemotherapy Drugs
In general, chemotherapy agents can be divided into three main categories based on their mechanism of action.
- Stop the synthesis of pre DNA molecule building blocks
These agents work in a number of different ways. DNA building blocks are folic acid, heterocyclic bases, and nucleotides, which are made naturally within cells. All of these agents work to block some step in the formation of nucleotides or deoxyribonucleotides (necessary for making DNA). When these steps are blocked, the nucleotides, which arethe building blocks of DNA and RNA, can not be synthesized. Thus the cells can not replicate because they can nnot make DNA without the nucleotides. Examples of drugs in this class include 1) methotrexate (Abitrexate®),2) fluorouracil (Adrucil®), 3) hydroxyurea (Hydrea®), and 4) mercaptopurine (Purinethol®).
- Directly damage the DNA in the nucleus of the cell
These agents chemically damage DNA and RNA. They disrupt replication of the DNA and either totally halt replication or cause the manufacture of nonsense DNA or RNA (i.e. the new DNA or RNA does not code for anything useful). Examples of drugs in this class include cisplatin (Platinol®) and 7) antibiotics - daunorubicin (Cerubidine®), doxorubicin (Adriamycin®), and etoposide (VePesid®).
- Effect the synthesis or breakdown of the mitotic spindles
Mitotic spindles serve as molecular railroads with "North and South Poles" in the cell when a cell starts to divide itself into two new cells. These spindles are very important because they help to split the newly copied DNA such that a copy goes to each of the two new cells during cell division. These drugs disrupt the formation of these spindles and therefore interrupt cell division. Examples of drugs in this class of 8) miotic disrupters include: Vinblastine (Velban®), Vincristine (Oncovin®) and Pacitaxel (Taxol®).
Enzyme L-asparaginase
In the 1950's a biochemical difference in metabolism related to the amino acid asparagine was found. Normal cells apparently can synthesize asparagine while leukemia cells cannot. If leukemia cells are deprived of asparagine, they will eventually die. In an almost unrecognized and parallel discovery, it was found that blood serum from guinea pigs and other South American rodents had antileukemia properties. The enzyme L-asparaginase was eventually identified as the anticancer agent. L-asparaginase was isolated and tested successfully on human leukemias. Eventually the enzyme asparaginase was also found and isolated from the bacteria, E. coli.
If the enzyme L-asparaginase is given to humans, various types of leukemias can be controlled. Tumor cells, more specifically lymphatic tumor cells, require huge amounts of asparagines to keep up with their rapid, malignant growth. This means they use both asparagine from the diet as well as what they can make themselves (which is limited) to satisfy their large asparagines demand. L-asparaginase is an enzyme that destroys asparagine external to the cell. Normal cells are able to make all the asparagine they need internally whereas tumor cells become depleted rapidly and die.The enzyme converts asparagine in the blood into aspartic acid by a deamination reaction. The leukemia cells are thus deprived of their supply of asparagine and will die.
Methotrexate
Methotrexate inhibits folic acid reductase which is responsible for the conversion of folic acid to tetrahydrofolic acid. At two stages in the biosynthesis of purines (adenine and guanine) and at one stage in the synthesis of pyrimidines (thymine, cytosine, and uracil), one-carbon transfer reactions occur which require specific coenzymes synthesized in the cell from tetrahydrofolic acid.
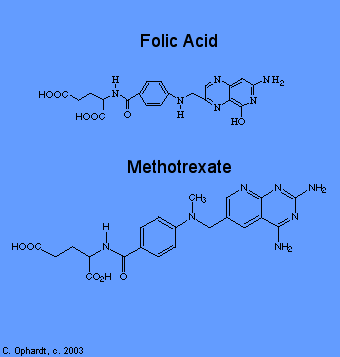
Tetrahydrofolic acid itself is synthesized in the cell from folic acid with the help of an enzyme, folic acid reductase. Methotrexate looks a lot like folic acid to the enzyme, so it binds to it thinking that it is folic acid. In fact, methotrexate looks so good to the enzyme that it binds to it quite strongly and inhibits the enzyme. Thus, DNA synthesis cannot proceed because the coenzymes needed for one-carbon transfer reactions are not produced from tetrahydrofolic acid because there is no tetrahydrofolic acid. Again, without DNA, no cell division.
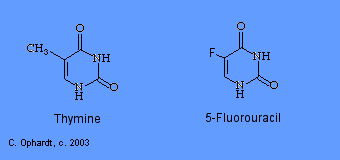
5-Fluorouracil
5-Fluorouracil (5-FU; Adrucil®, Fluorouracil, Efudex®, Fluoroplex®) is an effective pyrimidine antimetabolite. Fluorouracil is synthesized into the nucleotide, 5-fluoro-2-deoxyuridine. This product acts as an antimetabolite by inhibiting the synthesis of 2-deoxythymidine because the carbon - fluorine bond is extremely stable and prevents the addition of a methyl group in the 5-position. The failure to synthesize the thymidine nucleotide results in little or no production of DNA. Two other similar drugs include: gemcitabine (Gemzar®) and arabinosylcytosine (araC). They all work through similar mechanisms.
Contributors
Charles Ophardt (Professor Emeritus, Elmhurst College); Virtual Chembook

