Barbiturates and Benzodiazepines
- Page ID
- 413
Hypnotic and sedative drugs are non-selective, general depressants of the central nervous system. If the dose is relatively low, a sedative action results in a reduction in restlessness and emotional tension. A larger dose of the same drug produces a hypnotic sleep inducing effect. As the dosage is increased further, the result is anesthesia or death if the dosage is sufficiently high.
Introduction
The barbiturates once enjoyed a long period of extensive use as sedative-hypnotic drugs; however, except for a few specialized uses, they have been largely replaced by the much safer benzodiazepines. Barbiturates are central nervous system depressants and are similar, in many ways, to the depressant effects of alcohol. To date, there are about 2,500 derivatives of barbituric acid of which only 15 are used medically. The first barbiturate was synthesized from barbituric acid in 1864. The original use of barbiturates was to replace drugs such as opiates, bromides, and alcohol to induce sleep.
The hyponotic and sedative effects produced by barbiturates are usually ascribed to their interference of nerve transmission to the cortex. Various theories for the action of barbiturates include: changes in ion movements across the cell membrane; interactions with cholinergic and non cholinergic receptor sites; impairment of biochemical reactions which provide energy; and depression of selected areas of the brain. The structures of the barbiturates can be related to the duration of effective action. Although over 2000 derivatives of barbituric acid have been synthesized only about a dozen are currently used. All of the barbiturates are related to the structure of barbituric acid shown below.
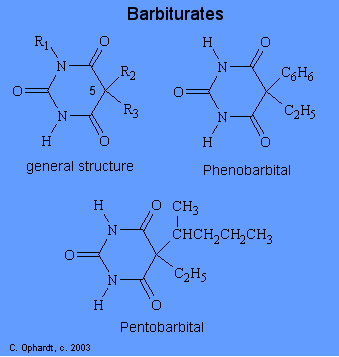
The duration of effect depends mainly on the alkyl groups attached to carbon # 5 which confer lipid solubility to the drug. The duration of effective action decreases as the total number of carbons at C # 5 increases. To be more specific, a long effect is achieved by a short chain and/or phenyl group. A short duration effect occurs when there are the most carbons and branches in the alkyl chains
Benzodiazepines
The term benzodiazepine refers to the portion of the structure composed of a benzene ring (A) fused to a seven-membered diazepine ring (B). However, since all of the important benzodiazepines contain a aryl substituent ring C) and a 1, 4-diazepine ring, the term has come to mean the aryl-1,4-benzodiazepines. There are several useful benzodiazepines available: chlordiazepoxide (Librium) and diazapam (Valium).
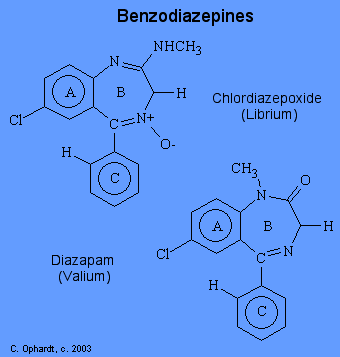
The actions of benzodiazepines are a result of increased activation of receptors by gamma-aminobutyric acid (GABA). Benzodiazepine receptors are located on the alpha subunit of the GABA receptor located almost exclusively on postsynaptic nerve endings in the CNS (especially cerebral cortex). Benzodiazepines enhance the GABA transmitter in the opening of postsynaptic chloride channels which leads to hyperpolarization of cell membranes. That is, they "bend" the receptor slightly so that GABA molecules attach to and activate their receptors more effectively and more often.
Contributors
Charles Ophardt (Professor Emeritus, Elmhurst College); Virtual Chembook

