Amino Acid Reactions
- Page ID
- 492
Amino acids react with each other in a typical acid-base neutralization reaction to form a salt.
The reaction is simply the transfer of the -H (positive ion) from the acid to the amine and the attraction of the positive and negative charges. The acid group becomes negative, and the amine nitrogen becomes positive because of the positive hydrogen ion.
For example in the graphic on the left - top, glycine (gly) and alanine (ala) may just interact in the zwitterion form by an attraction of the positive (amine) of the alanine and negative (carboxyl acid) charges to form the salt.
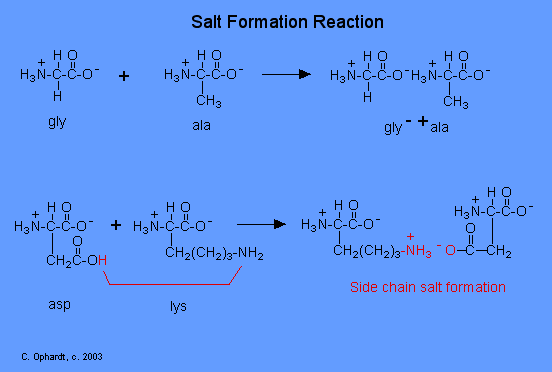
Salt formation of Side Chains
A more important interaction for protein tertiary structure is the interaction of the acid and base "side chains". If the amino acid has an extra acid or amine on the "side chain", these are used in the salt formation. For example in the left-bottom graphic, Aspartic acid (asp) has a side chain that forms a salt with the amine on the lysine (lys) side chain. The hydrogen ion (red) moves to the amine nitrogen resulting in the salt with the attraction of the positive and negative charges.
Disulfide Bridges and Oxidation-Reduction
The amino acid cysteine undergoes oxidation and reduction reactions involving the -SH (sulfhydryl group). The oxidation of two sulfhydryl groups results in the formation of a disulfide bond by the removal of two hydrogens. The oxidation of two cysteine amino acids is shown in the graphic on the left. An unspecified oxidizing agent (O) provides an oxygen which reacts with the hydrogen (red) on the -SH group to form water. The sulfurs (yellow) join to make the disulfide bridge. This is an important bond to recognize in protein tertiary structure.
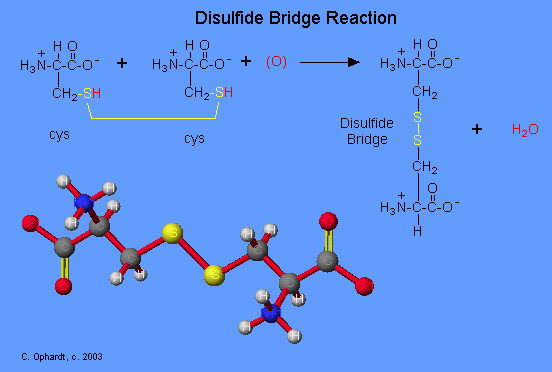
The reduction of a disulfide bond is the opposite reaction which again leads to two separate cysteine molecules. Remember that reduction is the addition of hydrogen.
Contributors
- Charles Ophardt, Professor Emeritus, Elmhurst College; Virtual Chembook

