Peptide Bonds
- Page ID
- 491
The formation of peptides is nothing more than the application of the amide synthesis reaction. By convention, the amide bond in the peptides should be made in the order that the amino acids are written. The amine end (N terminal) of an amino acid is always on the left, while the acid end (C terminal) is on the right. The reaction of glycine with alanine to form the dipeptide glyclalanine is written as shown in the graphic on the left. Oxygen (red) from the acid and hydrogens (red) on the amine form a water molecule. The carboxyl oxygen (green) and the amine nitrogen (green) join to form the amide bond.
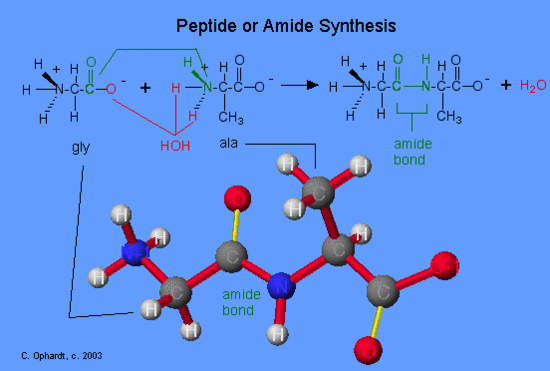
If the order of listing the amino acids is reversed, a different dipeptide is formed such as alaninylglycine.
Write the reactions for:
- ala + gly ---> Answer graphic
- phe + ser ----> Answer graphic
Salt Formation Contrast
The salt formation reaction is simply the transfer of the -H (positive ion) from the acid to the amine and the attraction of the positive and negative chagres. The acid group becomes negative, and the amine nitrogen becomes positive because of the positive hydrogen ion. No water is formed and there is only one product, the salt.
Backbone Peptide or Protein Structure
The structure of a peptide can be written fairly easily without showing the complete amide synthesis reaction by learning the structure of the "backbone" for peptides and proteins. The peptide backbone consists of repeating units of "N-H 2, CH, C double bond O; N-H 2, CH, C double bond O; etc. After the backbone is written, go back and write the specific structure for the side chains as represented by the "R" as gly-ala-leu for this example. The amine end (N terminal) of an amino acid is always on the left (gly), while the acid end (C terminal) is on the right (leu).
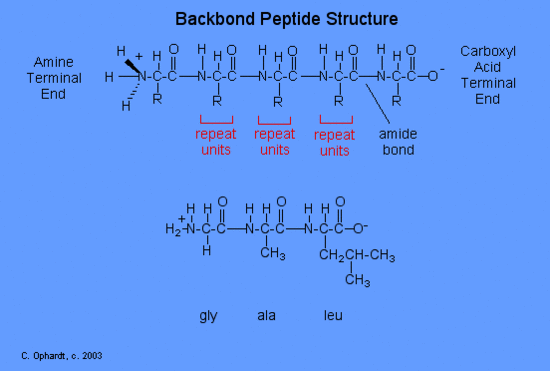
Write the tripeptide structure for val-ser-cys. First write the "backbone" and then add the specific side chains. Answer graphic
Write the structure for the tripeptide:
- a ) glu-cys-gly ---> Answer graphic
- b) phe-tyr-asn ---> Answer graphic
Glutathione
Glutathione is an important tripeptide present in significant concentrations in all tissues. It contains glutamic acid, cysteine, and glycine. What is unusual about the structure of glutathione (bottom figure below? Compare it to a "normal" tri peptide of glu-cys-gly (top graphic). Instead of the usual backbone, the carboxyl acid "side chain" is part of the backbone peptide structure. The normal carboxyl group is the so called side chain in this case.
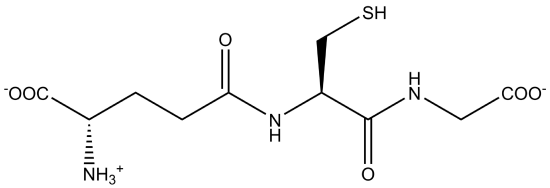
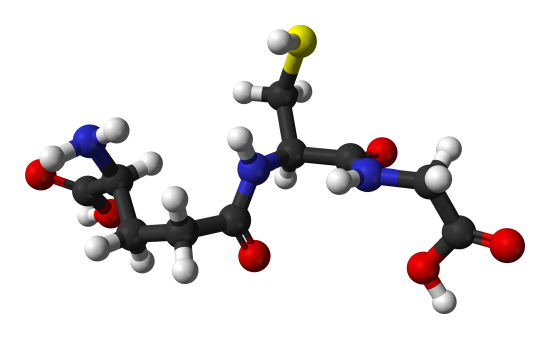
The function of glutathione is to protect cells from oxidizing agents which might otherwise damage them. The oxidizing agents react with the -SH group of cysteine of the glutathione instead of doing damage elsewhere. Many foreign chemicals get attached to glutathione, which is really acting as a detoxifying agent.
Contributors
Charles Ophardt (Professor Emeritus, Elmhurst College); Virtual Chembook

