The Triiodomethane (Iodoform) Reaction
- Page ID
- 3906
The triiodomethane (iodoform) reaction can be used to identify the presence of a CH3CH(OH) group in alcohols. There are two apparently quite different mixtures of reagents that can be used to do this reaction, but are chemically equivalent.
- Iodine and sodium hydroxide solution
This is chemically the more obvious method. Iodine solution is added to a small amount of an alcohol, followed by just enough sodium hydroxide solution to remove the color of the iodine. If nothing happens in the cold, it may be necessary to warm the mixture very gently. A positive result is the appearance of a very pale yellow precipitate of triiodomethane (previously known as iodoform): CHI3, which apart from its color, can also be recognized by its faintly "medical" smell. It is used as an antiseptic on the sort of sticky plasters you put on minor cuts, for example.
- Using potassium iodide and sodium chlorate(I) solutions
Sodium chlorate(I) is also known as sodium hypochlorite. Potassium iodide solution is added to a small amount of an alcohol, followed by sodium chlorate(I) solution. Again, if no precipitate is formed in the cold, it may be necessary to warm the mixture very gently. The positive result is the same pale yellow precipitate as before.
What the Iodoform Reaction Shows
A positive result is the formation of a pale yellow precipitate of triiodomethane (iodoform) - is given by an alcohol containing the grouping:
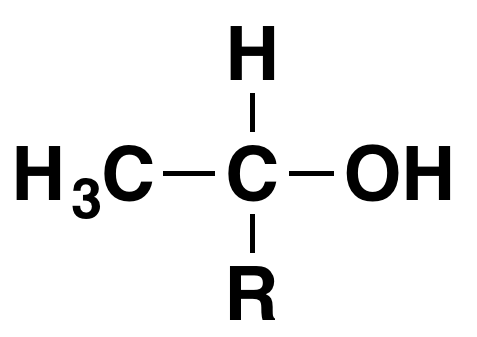
"R" can be a hydrogen atom or a hydrocarbon group (for example, an alkyl group). If "R" is hydrogen, then you have the primary alcohol ethanol, CH3CH2OH.
- Ethanol is the only primary alcohol to give the triiodomethane (iodoform) reaction.
- If "R" is a hydrocarbon group, then you have a secondary alcohol. Lots of secondary alcohols give this reaction, but those that do all have a methyl group attached to the carbon with the -OH group.
- No tertiary alcohols can contain this group because no tertiary alcohols can have a hydrogen atom attached to the carbon with the -OH group. No tertiary alcohols give the triiodomethane (iodoform) reaction.
Mechanism
We will assume the iodine/sodium hydroxide solution for the reaction:

This is being given as a flow scheme rather than full equations. The equations for the other two steps are given elsewhere.
Contributors
Jim Clark (Chemguide.co.uk)


