11.7: A look ahead: addition of carbon and hydride nucleophiles to carbonyls
- Page ID
- 965
We have seen in this chapter a number of reactions in which oxygen and nitrogen nucleophiles add to carbonyl groups. Other nucleophiles are possible in carbonyl addition mechanisms: in chapters 13 and 14, for example, we will examine in detail some enzyme-catalyzed reactions where the attacking nucleophile is a resonance stabilized carbanion.
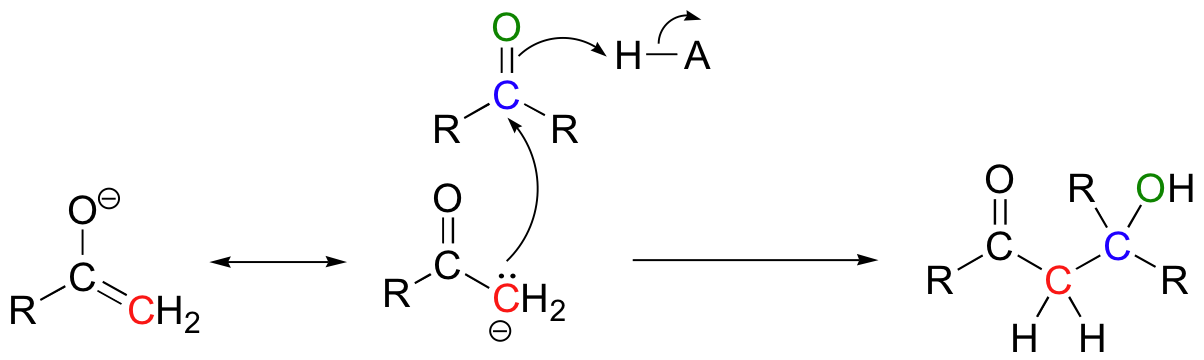
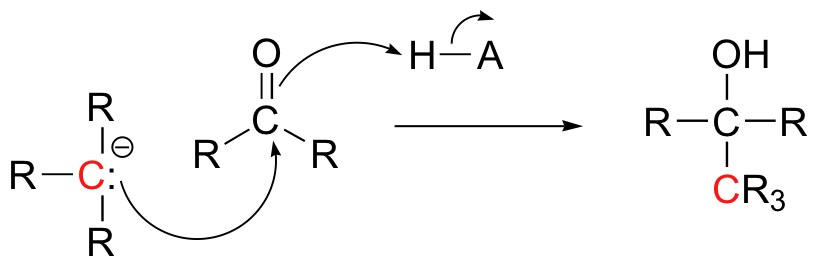
We will also see in chapter 13 some examples of laboratory synthesis reactions in which new carbon-carbon bonds are formed by first generating a species which reacts is if it were a carbanion, then allowing this powerful nucleophile to attack a carbonyl carbon on a second molecule.
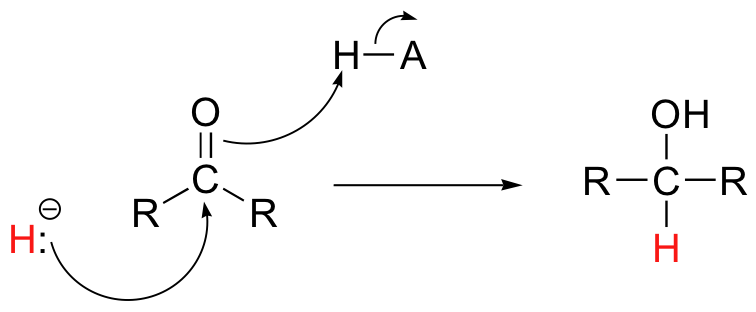
Then in chapter 16, we will see how the carbonyl groups on aldehydes and ketones can be converted to alcohols, both enzymatically as well as with synthetic reagents, through the addition of what is essentially a hydride (H-) ion.
. . . on to Chapter 11 problems . . .
. . . on to Chapter 12 . . .


