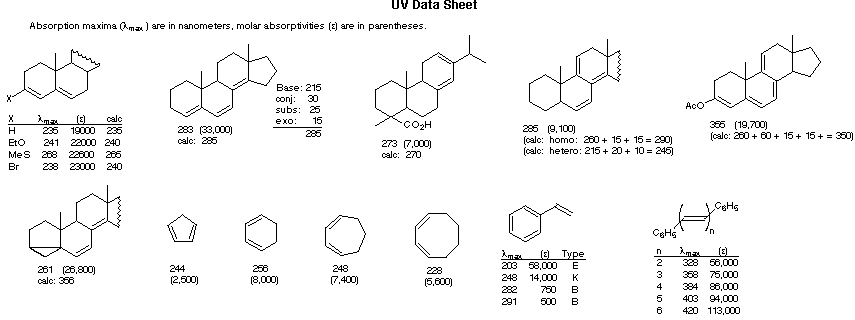
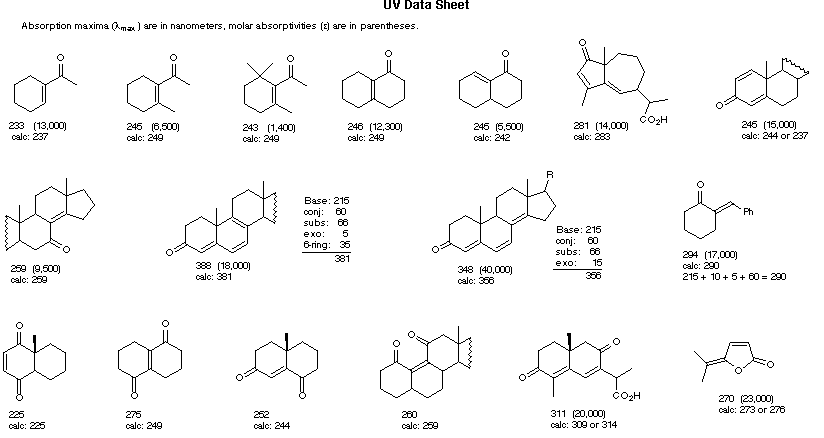
Empirical Rules for Absorption Wavelengths of Conjugated Systems
- Page ID
- 1245
Woodward-Fieser Rules for Calculating the λmax of Conjugated Dienes and Polyenes
|
Core Chromophore |
Substituent and Influence |
|---|---|
|
|
R- (Alkyl Group) .... +5 nm RO- (Alkoxy Group) .. +6 X- (Cl- or Br-) ......... +10 RCO2- (Acyl Group) .... 0 RS- (Sulfide Group) .. +30 R2N- (Amino Group) .. +60 Further π -Conjugation C=C (Double Bond) ... +30 C6H5 (Phenyl Group) ... +60 |
 Cyclohexadiene* 260 nm |
|
|
|
|
λmax (calculated) = Base (215 or 260) + Substituent Contributions
Woodward-Fieser Rules for Calculating the π → π* λmax of Conjugated Carbonyl Compounds
|
Core Chromophore |
Substituent and Influence |
|---|---|
 R = Alkyl 215 nm R = Alkyl 215 nmR = H 210 nm R = OR' 195 nm |
α- Substituent C6H5 (Phenyl Group) ... +60 |
 Cyclopentenone Cyclopentenone202 nm |
|
|
|
|
λmax (calculated) = Base + Substituent Contributions and Corrections
Examples
Contributors
- William Reusch, Professor Emeritus (Michigan State U.), Virtual Textbook of Organic Chemistry




 (i) Each exocyclic double bond adds 5 nm. In the example on the right, there are two exo-double bond components: one to ring A and the other to ring B.
(i) Each exocyclic double bond adds 5 nm. In the example on the right, there are two exo-double bond components: one to ring A and the other to ring B.