Delocalization of Electrons II
- Page ID
- 2007
Skills to Develop
- To introduce the concept of electron delocalization from the perspective of molecular orbitals, to understand the relationship between electron delocalization and resonance, and to learn the principles of electron movement used in writing resonance structures in Lewis notation, known as the curved arrow formalism.
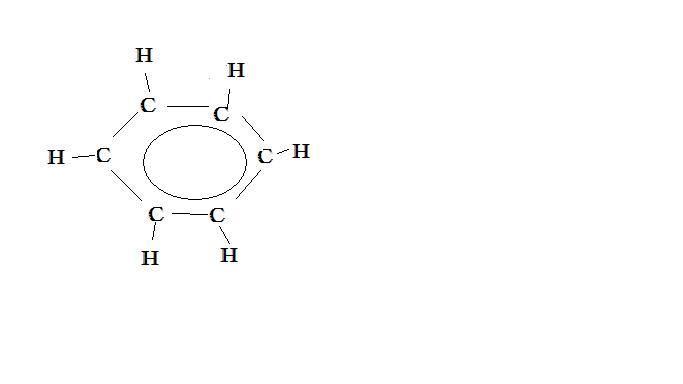
Electrons belonging to certain molecules are not attached to a particular atom or bond in that molecule. These electrons are said to be "delocalized" because they do not have a specific location (are not localized); they cannot be drawn in a simple Lewis structure. Rather, they exist in orbitals that include several atoms and/or bonds. You can imagine these orbitals as clouds surrounding parts of the molecule. Delocalization gives molecules resonance stability, stronger acidiy and based on the resonance stability, we can determine the range of absorbtion of ultraviolet and visible light. of a molecule in the light spectrum. The actual structure with delocalized electrons is called a resonance hybrid. According the valence-bond theory (resonance hybrids are structures whose forms are a mix of several representable forms. It is a mixture. This occurs because double or triple bonds can form between atoms). One of the many structures, with localized electrons, that are used to represent the hydrid molecule is called a resonance contributor. Electrons become delocalized in order to stabilize a structure. For example the benzene molecule, C6H6, delocalized electrons to stabilize its structure rather than having alternating double and single bonds, and is frequently drawn as a circle inside a hexagon to represent the shared electrons. Also, acetic acid, CH3COOH, has delocalized electrons that stabilize its conjugate base and thus make it acidic.
Delocalization Basics
Delocalization is characteristic of the molecular orbital theory concerning the structure of atoms. Rather than the lone pair of electrons contained in specific bonds (as in the valence-bond theory), the MO (molecular-orbital theory) theorizes that electrons exist in orbitals that are spread over the entire molecule. The MO theory explains molecules such as ozone and benzene, which cannot be drawn satisfactorily with one Lewis structure, and are therefore described as resonance hybrids. Molecular orbitals solve this issue through the concept of delocalized electrons.
When orbitals overlap, many of the orbitals are in hybrid states. Like delocalization, hybridization occurs to promote symmetry and stability. Delocalized electrons are often found in covalently bonded molecules that alternate single and multiple (usually double) bonds. Also, they frequently occur in aromatic systems and in mesoionic systems which means that they cannot exist in merely one resonance structure (they can and do exist in many resonance structures).
Delocalization matters for several reasons. One, spreading electron densities over a great area creates greater stability for the molecule. Spreading electrons creates charge distribution. Therefore, chemical reactions expected because of a hypothesized configuration may not occur, a stable product is formed more readily in a reaction. Delocalization of electrons also paves the way for conductivity while in spectroscopy, wavelenght absorbtion is also based on resonance stability and delocalization of electrons.
Resonance structures
Equivalent Lewis structures of the same molecule. The only difference between structures is where electrons are placed, and all options are equally probable. Common examples include ozone, nitrate, formate, and benzene.
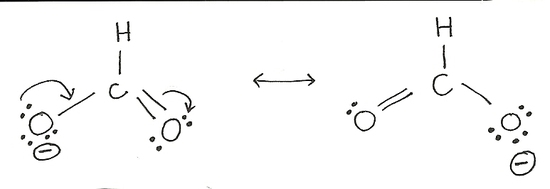
The structures of the formate ion above are equally likely. In both, all atoms have their octet, so the Lewis structure should be complete. However, instead of taking one form or another, the molecule incorporates both, creating resonance hybrids.
Resonance Contributors
The approximate structure of a molecule with localized electrons. Resonance contributors together are the contributors of the resonance hybrid. Sometimes there are multiple contributors and other times there are only two. Also, one contributor may contribute more to the overall hybrid than the other. The greater contributor is the most stable and thus the one that has delocalized electrons. Although, electronegativity and charge on molecules also affect the resonance contributors.
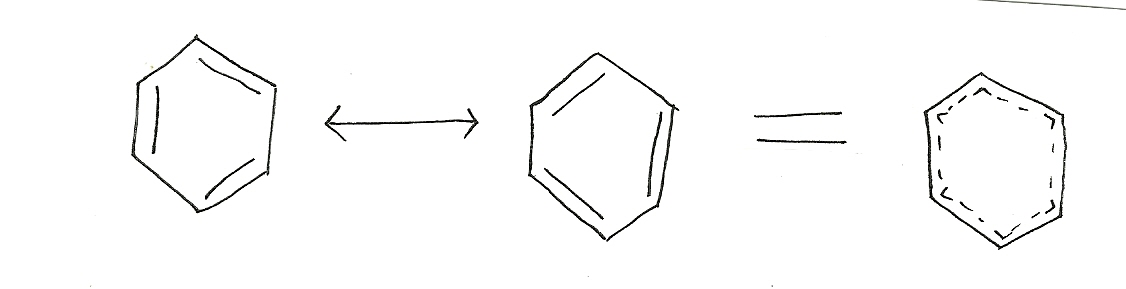
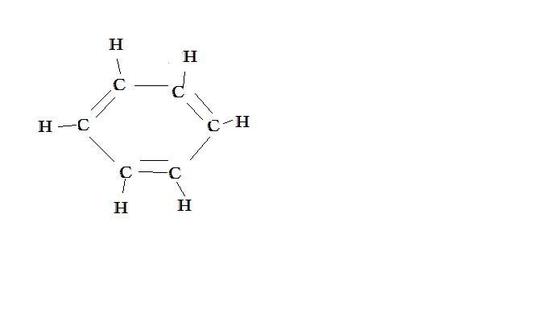
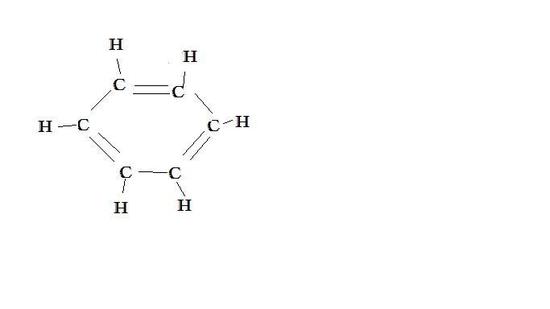
Benzene
resonance contributor resonance contributor resonance hybrid
Benzene has two resonance contributors that are equally stable. They both have delocalized electrons and do not have charges or different elecronegative atoms. Both equally contribute to the resonance hybrid ( picture above).
Resonance hybrid
A "compromise" between resonance structures. Molecules do not exist in both forms, or switch between forms, but spread electrons across multiple bonds to create symmetry. In reality, both bonds to oxygen in the formate ion are identical, indicating that the electrons are shared between the two bonds. For example, the formate ion resonance:
Hybridization
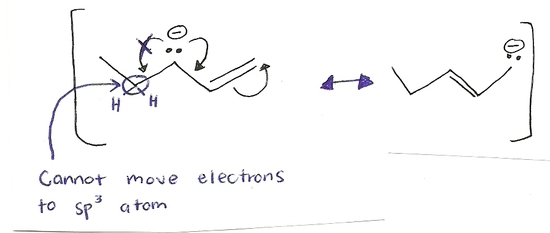
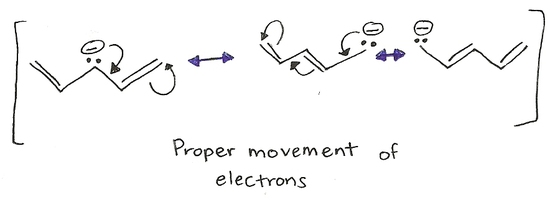
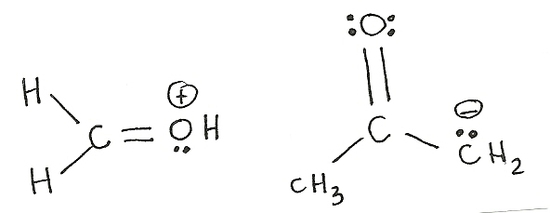
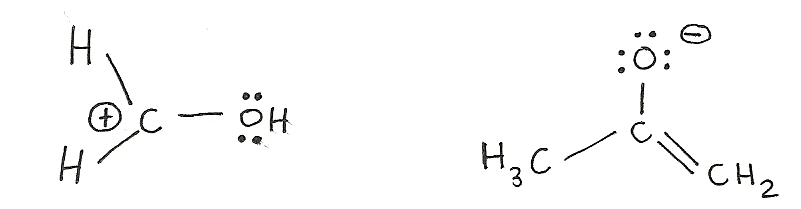
The process of mixing atomic orbitals to create new "hybrid" orbitals. These orbitals display qualities originating in the orbitals they were formed from. Electrons can only move to an carbon with an sp2hybridization. This is mainly because an sp2carbon does not have a full octet where an sp3carbon does have a full octet and cannot accomodate any more electrons. Also, only pi bonds and lone pairs move (double bonds) because they have an extra set of electrons that can be shared. This is shown in the example below.
The movement of electrons is towards an sp2carbon not an sp3. A lone pair or a double bond are the only possible movements of electrons. Generally, electrons move away from the negative charge and towards a positive charge. Electrons can do several movements of electrons in one structure (as shown on the right molecule.
Aromatic systems
Ring structures with continuous electron densities above and below planar ring(s). As a result, these structures display greater stability than is expected. An example is that of the Benzene ring in which the single and double bonds alternate in the molecule. Aromatic systems are more stable with lone pairs, unsaturated bonds and empty orbitals than what they would be with a conjugate alone (see example above, Benzene).
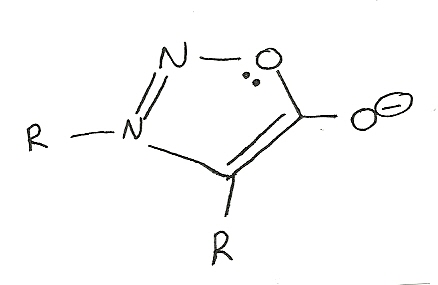
Mesoionic systems
Five or six membered cyclic compounds in which both negative and positive charge is delocalized. These systems contain carbon atoms and at least one other element. The structure below is a representation of what the systems are like. R represents an alkyl group of the form CH.
Delocalized electrons and pKa
pKa is the acidity of a molecule. The lower the pKa , the stronger the acid. Delocalization increases the stability so an increase in delocalization energy produces a more stable and more acidic molecule.
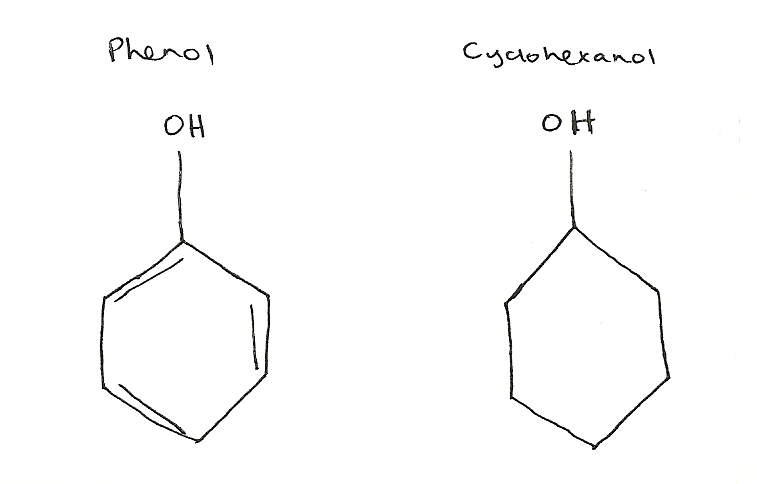
pKa= 10 pKa= 16
Phenol is a stronger acid because it is attached to a benzene ring that is delocalized and thus has resonance stability. Cyclohexanol is a weaker acid because it does not have resonance stability because it has localized electrons.
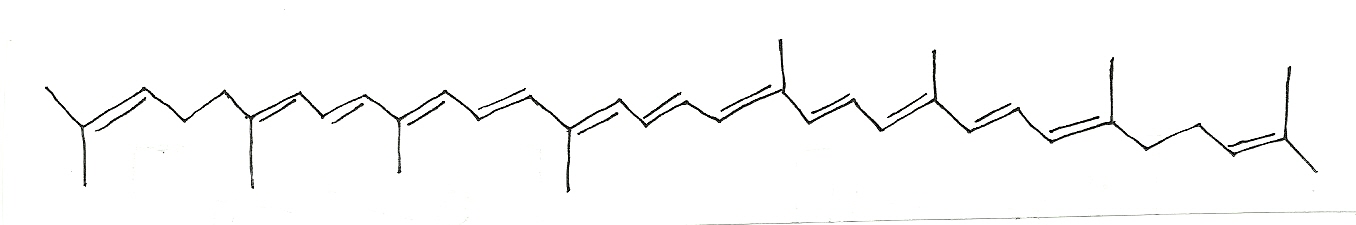
Delocalization and the light spectrum: The more the delocalized electrons, the longer the wavelength at which light is absorbed ( , lambda).
, lambda).
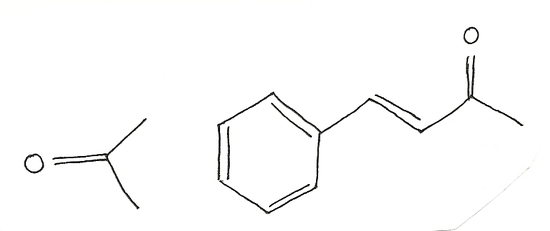
Acetone  =195nm Benzylidene
=195nm Benzylidene  =246.8nm Beta-Carotene
=246.8nm Beta-Carotene 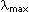 =455nm
=455nm
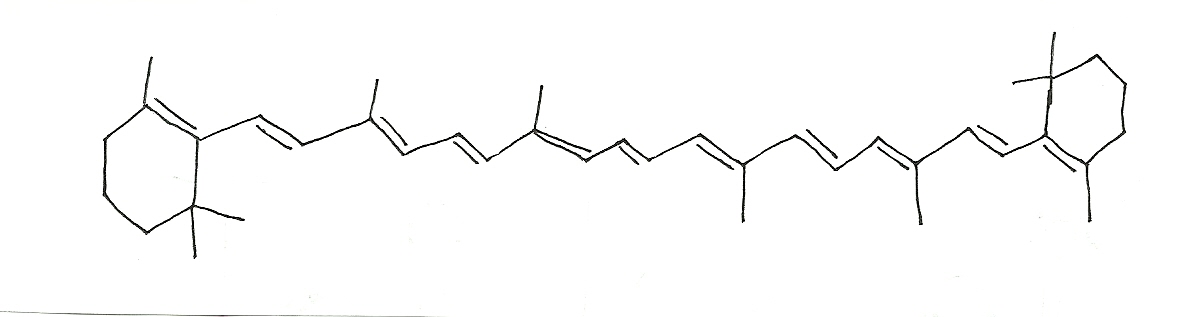
Lycopene 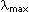 = 474nm
= 474nm
Rules for Drawing Electron Delocalization( Resonance contributors)
- Draw the Lewis structure of the molecule and we have the first resonance contributor.
- Only electrons move. Atoms never move.
- Only pi electrons and lone pair electrons can move, sigma bonds (single bonds) never move.
- The total number of electrons of the overall atom does not change (the charge). Therefore, all resonance contributors have the same charge.
- Electrons only move towards an sp2 carbon. That means that they can only move towards a positively charged atom or a double bonded one (usually a carbon).
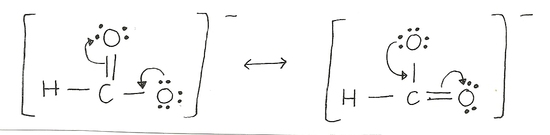
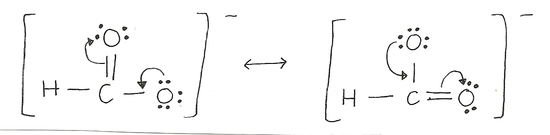
| Example: -COOH |
|---|
|
The arrows depict the movement of electrons. The resonance on the left moves the lone pair electrons away from the negave oxygen and toward a Carbon and to accomodate the new bond that has been formed and at the same time not violate the octet rule. The pi electrons move toward the electronegative oxygen in the form of lone pairs. Notice that the formal charge on the atoms change according to the electrons that it has. |
| Example 2: Benzene |
|---|
|
Recall, Benzene, C6H6, consists of a hexagonal ring of carbon atoms, each of which is bonded to a hydrogen and the adjacent carbons.According to the valence bond theory, the resonance structures of benzene appear as below (also drawn in example Benzene above).
However, rather than alternating single and double bonds, the electrons are actually shared throughout all six carbons, creating six 1.5 bonds, as shown below.
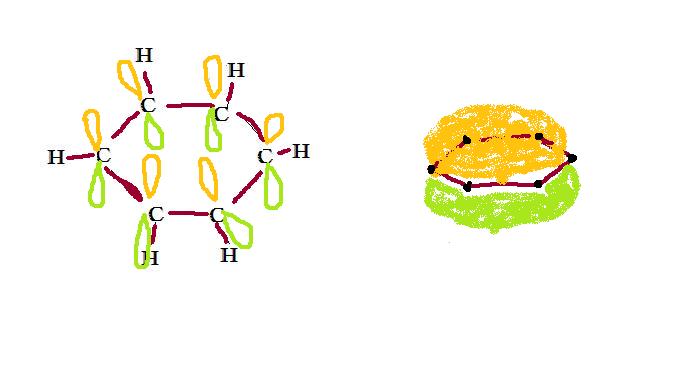
Carbon has full 2s orbitals and two electrons in the 2p orbital. In order to create a balanced structure, one electron from the 2s orbital moves to the 2p orbital, and then the sp2 hybrid orbital is formed. The sp2 orbitals form sigma bonds of the form carbon-carbon and carbon-hydrogen. The sigma bond between two carbons forms a single bond. These bonds use 24 of the 30 electrons in this molecule. (12 in the six bonds connecting carbons, and six in the bonds to hydrogen, where the hydrogen atoms provide the other electrons.) The remaining, unhybridized, 2p orbital (vacated during the formation of sp2 orbitals) forms delocalized pi bonds*. These bonds are not attached to specific carbons, but spread throughout the entire molecule. Thus, the remaining 6 electrons in the molecule allow half a bond to form between each carbon, resulting in 1.5 bonds throughout the structure. Delocalized electron fields can be imagined as doughnut-shaped regions above and below the hexagonal planar structure, as seen below. Electrostatic potential maps of the benzene molecule support this theory by displaying the accumulation of negative charge above and below the flat plane of the bonded atoms. Each electron is shared by all carbons in the ring. There are not enought electrons to form double bonds but the "extra" electrons strengthen all of the bonds equally. *Delocalized pi bonds are covalent chemical bonds in which the two lobes of one orbital overlap with the two lobes of another involved orbital |
Outside links
- Student Resources for General Chemistry, Delocalized Electrons: http://chemed.chem.wisc.edu/chempath...rons-1042.html
- Organic Chemistry; Fourth Edition, Electron Delocalization and Resonance: http://wps.prenhall.com/esm_bruice_o....cw/index.html
- Bishop, Mark. "Resonance." An Introduction to Chemistry - Bishop. 2010. Web. 05 June 2011. <http://preparatorychemistry.com/Bishop_Resonance.htm>.
- "ROCO Arrows: Curved Arrows." Reed College. Web. 05 June 2011. <http://academic.reed.edu/chemistry/r...ws/curved.html>.
- "CHM 331 : General Organic Chemistry." CHM 233 : Organic Chemistry I at Arizona State University. Arizona State University. Web. 05 June 2011. <http://chm233.asu.edu/notes/resonance/resonance.html>.
- "Acetone - Discussion and Encyclopedia Article. Who Is Acetone? What Is Acetone? Where Is Acetone? Definition of Acetone. Meaning of Acetone." Welcome to Knowledgerush. 2009. Web. 05 June 2011. <http://www.knowledgerush.com/kr/encyclopedia/Acetone/>.
- "Molecule Gallery - Alkenes." Angelo State University. Web. 05 June 2011. <http://www.angelo.edu/faculty/kboudr...00_alkenes.htm>.
- "Lycopene | 502-65-8." ChemicalBook---Chemical Search Engine. 2010. Web. 05 June 2011. <http://www.chemicalbook.com/Chemical..._CB5213951.htm>.
- "Illustrated Glossary of Organic Chemistry - Resonance Contributor Preference Rules." UCLA Chemistry and Biochemistry. Web. 05 June 2011. <http://www.chem.ucla.edu/harding/IGO...nce_rules.html>.
References
- Oxtoby, Gillis, and Campion. Principles of Modern Chemistry; Sixth Edition. Chapter 6.2. United States, 2008.
- Petrucci, Harwood, Herring, and Madura. General Chemistry: Principles and Modern Applications; Ninth Edition. Chapter 11-6. New Jersey, 2007.
- Super Review: Chemistry. Research and Education Association, Inc. New Jersey, 2008.
- Bruice, Paula Y. Essential Organic Chemistry. 2nd ed. Upper Saddle River: Pearson Education, 2010. 169-93. Print.
Practice Problems
1) Represent the structure of ozone, O3, according to the molecular orbital theory. Specifically what orbital(s) electrons occupy and what order bonds occur.
Answers
Ozone contains 18 electrons. According to VSEPR theory, this molecule should form trigonal-planar geometry (2 bonds + one pair = 3 electron density groups on central atom). 14 electrons are used in the hybridized sp2 orbital, which form sigma bonds. 4 exist as bonding electrons, and the remaining 10 exist as lone pairs distributes as appropriate. Three p orbitals are left unhybridized, and one of them is anti-bonding, and remains empty. Of the 4 electrons not yet assigned, two are used in each of the p orbitals that accept them. The bond order of these pi bonds is thus (2-0)/2=1. However, the pi bond is distributed between the two bonds, and each receives half of a pi bond. This molecule is represented below.
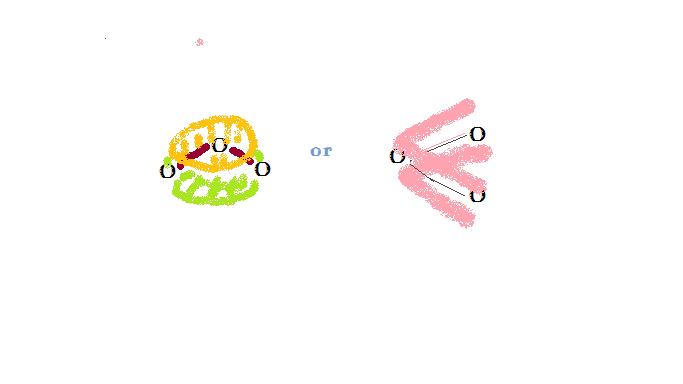
4) Why does a molecule portray delocalized electrons?
Answer: To gain stability which can lead to a more acidic molecule, a better resonance contributor, a greater absorber of the light spectrum and many other things.
5) What are some differences between the valence-bond theory and the molecular-orbital theory of bonding?
Contributors
- Brenda Mireles (UCD)