8.E: Chemical Reactions (Exercises)
- Page ID
- 44383
These are homework exercises to accompany the Textmap created for Chemistry: A Molecular Approach by Nivaldo Tro. Complementary General Chemistry question banks can be found for other Textmaps and can be accessed here. In addition to these publicly available questions, access to private problems bank for use in exams and homework is available to faculty only on an individual basis; please contact Delmar Larsen for an account with access permission.
Q8.31
Write a balanced chemical equation for the reaction of aluminum with phosphoric acid that produces hydrogen gas and aluminum phosphate.
Strategy
first write out equation with what state they are in
\[Al_{(s)} + H_3PO_{4(aq)} \rightarrow H_{2(g)} + AlPO_{4(aq)}\nonumber \]
then balance the equation
\[2 Al_{(s)} + 2 H_3PO_{4(aq)} \rightarrow 3 H_{2(g)} + 2 AlPO_{4(aq)}\nonumber \]
Hints
Remember charges when balancing equations
- Aluminum has a charge of 3+
- Phosphate has a charge of 3-
- Hydrogen a charge of 1+
www.youtube.com/watch?v=bGU6mQ6mC_s
Q8.35a
The unbalanced combustion equation for propane is:
\[C_{3}H_{8}(g) + O_{2}(g) \rightarrow CO_{2}(g) + H_{2}O(g)\nonumber \]
How many moles of water vapor will be produced when 4.65 moles of propane reacts with excess oxygen gas?
Solution
Step 1: Since the given equation is unbalanced, we must begin the solution by balancing the equation. Doing this will result in a balanced equation of:
\[C_{3}H_{8}(g) + 5O_{2}(g) \rightarrow 3CO_{2}(g) + 4H_{2}O(g)\nonumber \]
Step 2: The question states that oxygen gas is in excess, so we do not need to worry about finding our limiting reagent. Thus, we can find the ratio of water vapor to propane. This ratio, according the balanced reaction, is:
\[\dfrac{4mol\ H_{2}O(g)}{1mol\ C_{3}H_{8}(g)}\nonumber \]
Step 3: Using the ratio we found in Step 2, we can use dimensional analysis to find the moles of water vapor.
\[4.65mol\ C_{3}H_{8}(g)\cdot \dfrac{4mol\ H_{2}O(g)}{1mol\ C_{3}H_{8}(g)}\nonumber \]
After cancelling the propane:
\[4.65mol\ \cdot \dfrac{4mol\ H_{2}O(g)}{1mol\ }\nonumber \]
Step 5:
We are thus left with a simple multiplication problem where we can multiply 4.65 and 4.
After this, we find the number of moles of water vapor as:
\[{18.6mol\ H_{2}O(g)}\nonumber \]
Final Answer:
\[\mathit{18.6mol\ H_{2}O}\nonumber \]
Q8.35b
The unbalanced equation for the combustion of butane is shown below.
\[C_4H_{10} + O_2 \rightarrow H_2O + CO_2\nonumber \]
- Balance the equation.
- Calculate how many moles of \(C_4H_{10}\) are required to fully react 17.4 moles of \(O_2\).
Solutions
a.
1. Identify the various elements in the equation. Carbon (C), Hydrogen (H), and Oxygen (O) are the species being reacted in this case.
2. Determine how many atoms of each element are present on the respective sides of the equation.
| Chart 1 | Reactants | Products | |||||
|
Elements |
C |
H |
O |
O |
H |
C |
|
|
# atoms |
4 |
10 |
2 |
3 |
2 |
1 |
|
3. Chart 1 shows that the equation is not balanced. To balance the equation place integer coefficients in front of the molecules/elements to equal out the equation. It is not uncommon to have to try several different combination of coefficients to balance the equation. Hint: it is usually beneficial to balance oxygen last.
4. Balanced equation
\[2C_4H_{10} + 13O_2 \rightarrow 10H_2O + 8CO_2\nonumber \]
| Chart 2 | Reactants | Products | |||||
|
Elements |
C |
H |
O |
O |
H |
C |
|
|
# atoms |
8 |
20 |
26 |
26 |
20 |
8 |
|
Above is the balanced equation and chart showing the reactants and products balancing out.
b.)
1.) Now using the balanced equation, 2C4H10 + 13O2 → 10H2O + 8CO2 , it is possible to calculated how many moles of C4H10 are required to fully combust 17.4 moles of O2.
2.) The balanced equation tells us that it takes 13.0 moles of O2 to combust 2.00 moles of C4H10. Using this information we can create a proportion to solve the problem.
\[\dfrac{2.00\; mol\; C_{4}H_{10}}{13.0\; mol\; O_{2}}= \dfrac{?\; mol\; C_{4}H_{10}}{17.2\; mol\; O_{2}}\nonumber \]
\[(2.00\: mol\; C_{4}H_{10})(17.2\; mol\; O_{2})=(13\; mol\; O_{2})(?\; mol\; C_{4}H_{10})\nonumber \]
\[\dfrac{(2.00\: mol\; C_{4}H_{10})(17.2\; mol\; O_{2})}{(13.0\; moles\; O_{2})}=(?\; mol\; C_{4}H_{10})\nonumber \]
\[2.65\; mol\; C_{4}H_{10}\nonumber \]
Q8.37
How many moles of \(H_2\) form for each amount of \(C_2H_6\) reacted?
\[C_2H_{6(g)} \rightarrow C_2H_{2(g)}+2H_{2(g)}\nonumber \]
Strategy
- 2.1 mol C2H6
- mol C2H6 --> mol H2
- 3.4 mol C2H6
- mol C2H6 --> mol H2
- 5.68 g C2H6
- Convert g C2H6--> mol C2H6 ---> mol H2
- 2.35 g C2H6
- Convert g C2H6 --> mol C2H6 --> mol H2
Hint
In the equation, the number before the molecule is the amount of moles that specific molecule expresses. If it doesn’t have a number, that molecule has 1 mole
Solution
Hint: When converting from grams (g) to moles, divide by the number in the denominator (molar mass) to cancel out grams. Use the periodic table.
1) 2.1 mol C2H6 * 2 Mol H2/ 1 Mol C2H6= 4.2 mol H2
2) 3.4 mol C2H6 * 2 Mol H2/ 1 Mol C2H6= 6.8 mol H2
3) 5.68 g C2H6 *1 Mol C2H6/ 30 Mol C2H6 = .1893 mol C2H6
0.1893 mol C2H6 *2 Mol H2/ 1 Mol
4) 2.35 g C2H6*1 Mol C2H6/ 1 Mol C2H6= .0783 mol C2H6
0.0783 mol C2H6*2 Mol H2/ 1 Mol C2H6 H2
Q8.41
Photosynthesis is a chemical reaction in plants used to produce oxygen and glucose. If a plant uses 6.3g CO2, how much water (in grams) is needed to complete the reaction? How much glucose will be formed?
\[6CO_{2}+6H_{2}O+light\rightarrow C_{6}H_{12}O_{6}+6O_{2}\nonumber \]
Solution/ part 1
To solve this you must first convert grams of CO2 to moles of CO2. From there you cross the mole bridge, which is the mole ratio between the molecules, this simply means that you will be going from moles of one compound to moles of a different compound. Finally, you must convert the moles of H20 to grams of H20. Then, you will plug all of those numbers into your calculator to get your solution.
Step 1 The first step to solving this problem is to convert the grams of CO2 to moles of CO2. To do this you will divide 1 mol CO2 by the molar mass of CO2. You find the molar mass by looking on the periodic table. You then multiply 6.3g CO2 by the value you found which is \[\dfrac{1molCO_{2}}{44gCO_{2}}.\nonumber \]
\[6.3gCO_{2}\dfrac{1molCO_{2}}{44gCO_{2}}\nonumber \]
Step 2 Next, you need to cross the mole bridge. This is where you show the mole ratio between the molecules. This simply means that you will be going from moles of CO2 to moles of H20. You do this because you ae trying to solve for how much water is needed to complete the reaction and you started with CO2. The mole ratio of CO2 to H20 is 6:6. If you look at the equation you will see that. You need to divide the moles H20 by moles of CO2. You will take step 1 and multiply it with what you have found here which is \[\dfrac{6molH_{2}O}{6molCO_{2}}\nonumber \]. This will give you
\[6.3gCO_{2}*\dfrac{1molCO_{2}}{44gCO_{2}}*\dfrac{6molH_{2}O}{6molCO_{2}}\nonumber \]
Step 3 Now you need to convert mol H20 to grams of H20. To do that you need to multiply by the molar mass of H20. Once again, you can find the molar mass on the periodic table. The molar mass of H20 is 18 grams per mole.\[\dfrac{18gH_{2}O}{1molH_{2}O}\nonumber \]. Now that you have this you will this value and multiply it by what you found in step 2. This will give you
\[6.3gCO_{2}*\dfrac{1molCO_{2}}{44gCO_{2}}*\dfrac{6molH_{2}O}{6molCO_{2}}*\dfrac{18gH_{2}O}{1molH_{2}O}\nonumber \]
Step 4Now that you have all your values you just plug them into your calculator and get a value of 2.5773g H20. Since you have 2 sig figs in the question your answer should have 2 sig figs, so you get an answer of 2.6g H2O.
Solution/ part 2
To solve this part you must convert the grams of CO2 to moles of CO2. From there you cross the mole bridge, which is the mole ratio between the molecules. Finally you must convert the moles of glucose to grams of glucose. Then you will plug all of those numbers into your calculator to get your solution.
For this part you are trying to solve for how much glucose will be formed given 6.3g CO2.
Step 1 The first step to solving this problem is to convert the grams of CO2 to moles of CO2. To do this you will divide 1 mol CO2 by the molar mass of CO2. You find the molar mass by looking on the periodic table. You then multiply 6.3g CO2 by the value you found which is \[\dfrac{1molCO_{2}}{44gCO_{2}}.\nonumber \]
\[6.3gCO_{2}\dfrac{1molCO_{2}}{44gCO_{2}}\nonumber \]
Step 2 Next, you need to cross the mole bridge. This is where you show the mole ratio between the molecules. This simply means that you will be going from moles of CO2 to moles of C6H12O6. You do this because you ae trying to solve for how much glucose will be formed with the reaction and you started with CO2.The mole ratio of CO2 to C6H12O6 is 6:1. This is given in the equation. Now you need to divide moles of C6H12O6 by the moles of CO2. Which is \[\dfrac{1molC_{6}H_{12}O_{6}}{6molCO_{2}}\nonumber \]. Then you take Step 1 and multiply is by this value to give you
\[6.3gCO_{2}\dfrac{1molCO_{2}}{44gCO_{2}}*\dfrac{1molC_{6}H_{12}O_{6}}{6molCO_{2}}\nonumber \]
Step 3 Now you need to convert moles to grams. To do that you need to multiply by the molar mass of C6H12O6 which is 180 grams per mole.\[\dfrac{180gC_{6}H_{12}O_{6}}{1molC_{6}H_{12}O_{6}}\nonumber \]. Then you multiply this value by step 2 to give you
\[6.3gCO_{2}\dfrac{1molCO_{2}}{44gCO_{2}}*\dfrac{1molC_{6}H_{12}O_{6}}{6molCO_{2}}*\dfrac{180gC_{6}H_{12}O_{6}}{1molC_{6}H_{12}O_{6}}\nonumber \]
Step 4 Now that you have all your values you just plug them into your calculator and get a value of 4.295g C6H12O6. Since you had two sig figs in the question, there will be two sig figs. So your answer will be 4.2g C6H12O6 formed.
Q8.53
For the reactions listed, calculate the theoretical yield for the products in grams using the listed initial amounts of the reactants.
- 3g Al, 4g Cl
- 9g Al, 18g Cl
- 0.5g Al, 2g Cl
Strategy:
- Find the molar mass of Al3+, Cl-, and AlCl3
- Convert the initial amounts of the reactants in grams to moles of reactants (mass to mole conversion)
- Convert the moles of reactants to moles of product (mole to mole conversion)
- Convert the moles of product to mass of product in grams (mole to mass conversion)
Hints:
1. To convert from Mass A to Mass B to find grams of products, use the formula 
2. Theoretical yield - the amount predicted by a stoichometric calculation based on the number of moles of the reactants present to form the products
3. Keep in mind that the limiting reagent is whatever reactant gives you the smallest number because you can't create more than the limiting reagent can form, so the theoretical yield is the same as what the limiting reagent produces.
Solution:
a. 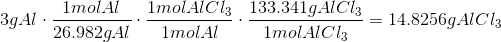
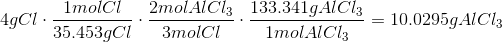
The Theoretical Yield with 3g Al and 4g CL is 10.0295g AlCl3 with Cl being the limiting reagent.
b. 
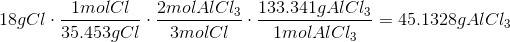
The Theoretical Yield with 9g Al and 18g Cl is 44.4767g AlCl3 with Al being the limiting reagent.
c. 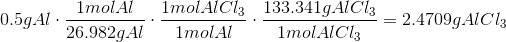
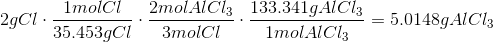
The Theoretical Yield with0.5g Al and 2g Cl is2.4709g AlCl3 with Al being the limiting reagent.

