Physical Properties of Period 3 Oxides
- Page ID
- 545
This page explains the relationship between the physical properties of the oxides of Period 3 elements (sodium to chlorine) and their structures. Argon is obviously omitted because it does not form an oxide.
The oxides
The oxides we'll be looking at are:
| Na2O | MgO | Al2O3 | SiO2 | P4O10 | SO3 | Cl2O7 |
| P4O6 | SO2 | Cl2O |
Those oxides in the top row are known as the highest oxides of the various elements. These are the oxides where the Period 3 elements are in their highest oxidation states. In these oxides, all the outer electrons in the Period 3 element are being involved in the bonding - from just the one with sodium, to all seven of chlorine's outer electrons.
The structures
The trend in structure is from the metallic oxides containing giant structures of ions on the left of the period via a giant covalent oxide (silicon dioxide) in the middle to molecular oxides on the right.
Melting and boiling points
The giant structures (the metal oxides and silicon dioxide) will have high melting and boiling points because a lot of energy is needed to break the strong bonds (ionic or covalent) operating in three dimensions. The oxides of phosphorus, sulfur and chlorine consist of individual molecules; some are small and simple and others are polymeric.
The attractive forces between these molecules will be van der Waals dispersion and dipole-dipole interactions. These vary in size depending on the size, shape and polarity of the various molecules - but will always be much weaker than the ionic or covalent bonds you need to break in a giant structure. These oxides tend to be gases, liquids or low melting point solids.
Electrical conductivity
None of these oxides has any free or mobile electrons. That means that none of them will conduct electricity when they are solid. The ionic oxides can, however, undergo electrolysis when they are molten. They can conduct electricity because of the movement of the ions towards the electrodes and the discharge of the ions when they get there.
The metallic oxides
The structures
Sodium, magnesium and aluminium oxides consist of giant structures containing metal ions and oxide ions. Magnesium oxide has a structure just like sodium chloride. The other two have more complicated arrangements.
Melting and boiling points
There are strong attractions between the ions in each of these oxides and these attractions need a lot of heat energy to break. These oxides therefore have high melting and boiling points.
Electrical conductivity
None of these conducts electricity in the solid state, but electrolysis is possible if they are molten. They conduct electricity because of the movement and discharge of the ions present. The only important example of this is in the electrolysis of aluminium oxide in the manufacture of aluminium .
Whether you can electrolyse molten sodium oxide depends, of course, on whether it actually melts instead of subliming or decomposing under ordinary circumstances. If it sublimes, you will not get any liquid to electrolyse! Magnesium and aluminium oxides have melting points far too high to be able to electrolyse them in a simple lab.
Silicon dioxide (silicon(IV) oxide)
The structure
The electronegativity of the elements increases as you go across the period, and by the time you get to silicon, there is not enough electronegativity difference between the silicon and the oxygen to form an ionic bond. Silicon dioxide is a giant covalent structure. There are three different crystal forms of silicon dioxide. The easiest one to remember and draw is based on the diamond structure. Crystalline silicon has the same structure as diamond. To turn it into silicon dioxide, all you need to do is to modify the silicon structure by including some oxygen atoms.

Notice that each silicon atom is bridged to its neighbours by an oxygen atom. Don't forget that this is just a tiny part of a giant structure extending in all 3 dimensions.
If you want to be fussy, the Si-O-Si bond angles are wrong in this diagram. In reality the "bridge" from one silicon atom to its neighbour is not in a straight line, but via a "V" shape (similar to the shape around the oxygen atom in a water molecule). It's extremely difficult to draw that convincingly and tidily in a diagram involving this number of atoms. The simplification is perfectly acceptable.
Melting and boiling points: Silicon dioxide has a high melting point - varying depending on what the particular structure is (remember that the structure given is only one of three possible structures), but they are all around 1700°C. Very strong silicon-oxygen covalent bonds have to be broken throughout the structure before melting occurs. Silicon dioxide boils at 2230°C.
Because you are talking about a different form of bonding, it doesn't make sense to try to compare these values directly with the metallic oxides. What you can safely say is that because the metallic oxides and silicon dioxide have giant structures, the melting and boiling points are all high.
Electrical conductivity: Silicon dioxide does not have any mobile electrons or ions and hence does not conduct electricity either as a solid or a liquid.
The molecular oxides
Phosphorus, sulfur and chlorine all form oxides which consist of molecules. Some of these molecules are fairly simple - others are polymeric. We are just going to look at some of the simple ones. Melting and boiling points of these oxides will be much lower than those of the metal oxides or silicon dioxide. The intermolecular forces holding one molecule to its neighbors will be van der Waals dispersion forces or dipole-dipole interactions. The strength of these will vary depending on the size of the molecules. None of these oxides conducts electricity either as solids or as liquids. None of them contains ions or free electrons.
The Phosphorus Oxides
Phosphorus has two common oxides, phosphorus(III) oxide, P4O6, and phosphorus(V) oxide, P4O10.
Phosphorus(III) oxide
Phosphorus(III) oxide is a white solid, melting at 24°C and boiling at 173°C. The structure of its molecule is best worked out starting from a P4 molecule which is a little tetrahedron.

Pull this apart so that you can see the bonds . . .
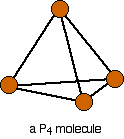
. . . and then replace the bonds by new bonds linking the phosphorus atoms via oxygen atoms. These will be in a V-shape (rather like in water), but you probably wouldn't be penalised if you drew them on a straight line between the phosphorus atoms in an exam.
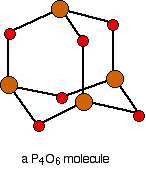
The phosphorus is using only three of its outer electrons (the 3 unpaired p electrons) to form bonds with the oxygens.
Phosphorus(V) oxide
Phosphorus(V) oxide is also a white solid, subliming (turning straight from solid to vapour) at 300°C. In this case, the phosphorus uses all five of its outer electrons in the bonding. Solid phosphorus(V) oxide exists in several different forms - some of them polymeric. We are going to concentrate on a simple molecular form, and this is also present in the vapor. This is most easily drawn starting from P4O6. The other four oxygens are attached to the four phosphorus atoms via double bonds.
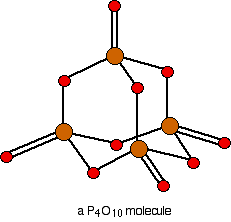
If you look carefully, the shape of this molecule looks very much like the way we usually draw the repeating unit in the diamond giant structure. Don't confuse the two, though! The \(P_4O_{10}\) molecule stops here. This is not a little bit of a giant structure - it's all there is. In diamond, of course, the structure just continues almost endlessly in three dimensions.
The Sulfur Oxides
Sulfur has two common oxides, sulfur dioxide (sulfur(IV) oxide), SO2, and sulfur trioxide (sulfur(VI) oxide), SO3.
sulfur dioxide
Sulfur dioxide is a colourless gas at room temperature with an easily recognised choking smell. It consists of simple SO2 molecules.
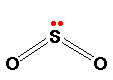
The sulfur uses 4 of its outer electrons to form the double bonds with the oxygen, leaving the other two as a lone pair on the sulfur. The bent shape of SO2 is due to this lone pair.
sulfur trioxide
Pure sulfur trioxide is a white solid with a low melting and boiling point. It reacts very rapidly with water vapour in the air to form sulfuric acid. That means that if you make some in the lab, you tend to see it as a white sludge which fumes dramatically in moist air (forming a fog of sulfuric acid droplets). Gaseous sulfur trioxide consists of simple SO3 molecules in which all six of the sulfur's outer electrons are involved in the bonding.
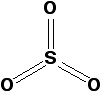
There are various forms of solid sulfur trioxide. The simplest one is a trimer, S3O9, where three SO3 molecules are joined up and arranged in a ring.
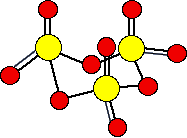
There are also other polymeric forms in which the SO3 molecules join together in long chains. For example:
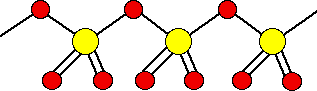
It is difficult to draw this convincingly. In fact, on each sulfur atom, one of the double bonded oxygens is coming out of the diagram towards you, and the other one is going back in away from you.
The fact that the simple molecules join up in this way to make bigger structures is what makes the sulfur trioxide a solid rather than a gas.
The chlorine oxides
Chlorine forms several oxides. Here we are just looking at two of them : chlorine(I) oxide (Cl2O) and chlorine(VII) oxide (Cl2O7).
Chlorine(I) oxide
Chlorine(I) oxide is a yellowish-red gas at room temperature. It consists of simple small molecules.

There's nothing in the least surprising about this molecule and it's physical properties are just what you would expect for a molecule this size.
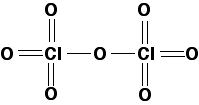
Chlorine(VII) oxide
In chlorine(VII) oxide, the chlorine uses all of its seven outer electrons in bonds with oxygen. This produces a much bigger molecule, and so you would expect its melting point and boiling point to be higher than chlorine(I) oxide. Chlorine(VII) oxide is a colourless oily liquid at room temperature. In the diagram, for simplicity I have drawn a standard structural formula. In fact, the shape is tetrahedral around both chlorines, and V-shaped around the central oxygen.
Problems
I intended at this point to quote values for each of the oxides, hoping to show that the melting and boiling points increase as the charges on the positive ion increase from 1+ in sodium to 3+ in aluminium. You would expect that the greater the charge, the greater the attractions. Unfortunately, the oxide with the highest melting and boiling point is magnesium oxide, not aluminium oxide! So that theory bit the dust!
The reason for this probably lies in the increase in electronegativity as you go from sodium to magnesium to aluminium. That would mean that the electronegativity difference between the metal and the oxygen is decreasing. The smaller difference means that the bond won't be so purely ionic. It is also likely that molten aluminium oxide contains complex ions containing both aluminium and oxygen rather than simple aluminium and oxide ions. All this means, of course, that you aren't really comparing like with like - so wouldn't necessarily expect a neat trend.
The other problems I came across lie with sodium oxide. Most sources say that this sublimes (turns straight from solid to vapour) at 1275°C. However, the usually reliable Webelements gives a melting point of 1132°C followed by a decomposition temperature (before boiling) of 1950°C. Other sources talk about it decomposing (to sodium and sodium peroxide) above 400°C. I have no idea what the truth of this is - although I suspect that the Webelements melting point value is probably for a pressure above atmospheric pressure (although it doesn't say so).


