Chemistry of Sulfur (Z=16)
- Page ID
- 573
- Describe the chemistry of the oxygen group.
- Give the trend of various properties.
- Remember the names of Group 16 elements.
- Explain the Frasch process.
- Describe properties and applications of \(\mathrm{H_2SO_4}\).
- Explain properties and applications of \(\mathrm{H_2S}\).
Sulfur is a chemical element that is represented with the chemical symbol "S" and the atomic number 16 on the periodic table. Because it is 0.0384% of the Earth's crust, sulfur is the seventeenth most abundant element following strontium. Sulfur also takes on many forms, which include elemental sulfur, organo-sulfur compounds in oil and coal, H2S(g) in natural gas, and mineral sulfides and sulfates. This element is extracted by using the Frasch process (discussed below), a method where superheated water and compressed air are used to draw liquid sulfur to the surface. Offshore sites, Texas, and Louisiana are the primary sites that yield extensive amounts of elemental sulfur. However, elemental sulfur can also be produced by reducing H2S, commonly found in oil and natural gas. For the most part, though, sulfur is used to produce SO2(g) and H2SO4.
Known from ancient times (mentioned in the Hebrew scriptures as brimstone) sulfur was classified as an element in 1777 by Lavoisier. Pure sulfur is tasteless and odorless with a light yellow color. Samples of sulfur often encountered in the lab have a noticeable odor. Sulfur is the tenth most abundant element in the known universe.
| Atomic Number | 16 |
| Atomic Symbol | S |
| Atomic Weight | 32.07 grams per mole |
| Structure | orthorhombic |
| Phase at room temperature | solid |
| Classification | nonmetal |
Physical Properties of Sulfur
Sulfur has an atomic weight of 32.066 grams per mole and is part of group 16, the oxygen family. It is a nonmetal and has a specific heat of 0.706 J g-1 oC-1. The electron affinity is 200 kJ mol-1 and the electronegativity is 2.58 (unitless). Sulfur is typically found as a light-yellow, opaque, and brittle solid in large amounts of small orthorhombic crystals. Not only does sulfur have twice the density of water, but it is also insoluble in water. On the other hand, sulfur is highly soluble in carbon disulfide and slightly soluble in many common solvents. Sulfur can also vary in color and blackens upon boiling due to carbonaceous impurities. Even as little as 0.05% of carbonaceous matter darkens sulfur significantly.
Most sulfur is recovered directly as the element from underground deposits by injecting super-heated water and piping out molten sulfur (sulfur melts at 112o C). Compared to other elements, sulfur has the most allotropes. While the S8 ring is the most common allotrope, there are 6 other structures with up to 20 sulfur atoms per ring.
- Under appropriate conditions, sulfur vapor can contain \(S\), \(S_2\), \(S_4\), \(S_6\), and \(S_8\).
- At room temperature, rhombic sulfur (Sα) is a stable solid comprising cyclic \(S_8\) molecules.
- At 95.5 °C, rhombic sulfur becomes monoclinic sulfur (Sβ). The crystal structure found in monoclinic sulfur differs from that of rhombic sulfur. Monoclinic sulfur is also made up of \(S_8\)molecules.
- Monoclinic sulfur becomes liquid sulfur (Sλ) at 119 °C. Liquid sulfur is a straw-colored liquid made up of \(S_8\) molecules and other cyclic molecules containing a range of six to twenty atoms.
- At 160 oC, this becomes a dark, viscous liquid called liquid sulfur (Sμ). The molecules are still made up of eight sulfur atoms, but the molecule opens up and transforms from a circle into a long spiral-chain molecule.
- At 180 °C, the chain length and viscosity reach their maximum. Chains break and viscosity decreases at temperatures that exceed 180 °C.
- Sulfur vapor is produced when liquid boils at 445 °C. In the vapor that is produced, \(S_8\) molecules dominate, but as the vapor continues to heat up, the molecules break up into smaller groups of sulfur atoms.
- To produce plastic sulfur, S is poured into cold water. Plastic sulfur is rubberlike and is made up of long, spiral-chain molecules. If plastic sulfur sits for long, it will reconvert to rhombic sulfur.
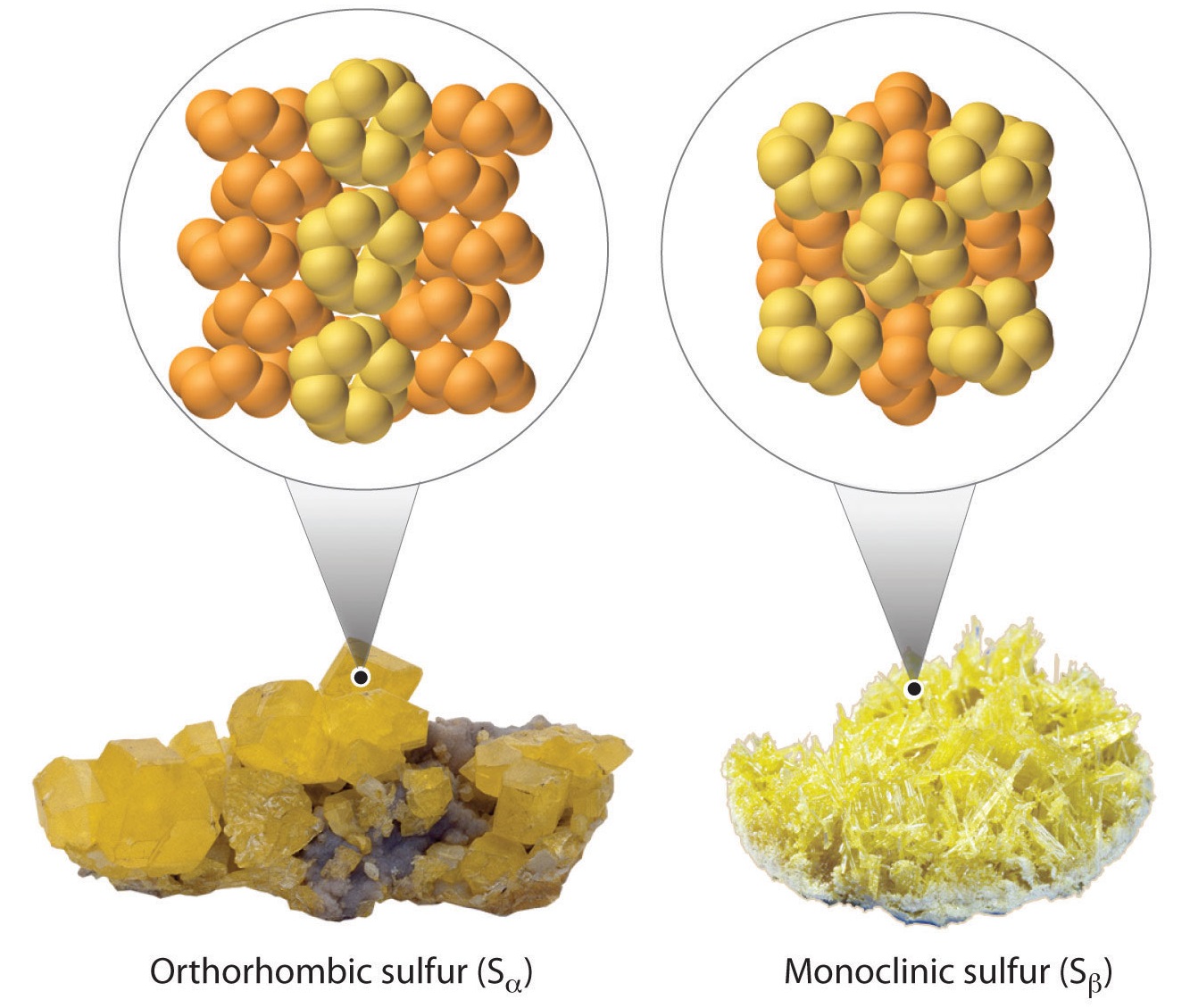
While oxygen has fewer allotropes than sulfur, including \(\ce{O}\), \(\ce{O_2}\), \(\ce{O_3}\), \(\ce{O_4}\), \(\ce{O_8}\), metallic \(\ce{O}\) (and four other solid phases), many of these actually have a corresponding sulfur variant. However, sulfur has more tendency to catenate (the linkage of atoms of the same element into longer chains). Here are the values of the single and double bond enthalpies:
\[\begin{array}{c|r} \ce {O-O} & \ce{142\ kJ/mol} \\ \ce {S–S} & \ce{268\ kJ/mol} \\ \ce {O=O} & \ce{499\ kJ/mol} \\ \ce {S=S} & \ce{352\ kJ/mol} \\ \end{array} \nonumber \]
This means that \(\ce{O=O}\) is stronger than \(\ce{S=S}\), while \(\ce{O–O}\) is weaker than \(\ce{S–S}\). So, in sulfur, single bonds are favored and catenation is easier than in oxygen compounds. It seems that the reason for the weaker \(\ce{S=S}\) double bonds has its roots in the size of the atom: it's harder for the two atoms to come to a small enough distance, so that the \(p\) orbital overlap is small and the \(\pi\) bond is weak. This is attested by looking down the periodic table: \(\ce{Se=Se}\) has an even weaker bond enthalpy of \(\ce{272 kJ/mol}\).
What happens when the solid sulfur melts? The \(\ce{S8}\) molecules break up. When suddenly cooled, long chain molecules are formed in the plastic sulfur which behave like rubber. Plastic sulfur transforms into rhombic sulfur over time.
Compounds
Reading the following reactions, figure out and notice the change of the oxidation state of \(\ce{S}\) in the reactants and products. Common oxidation states of sulfur are -2, 0, +4, and +6. Sulfur (brimstone, stone that burns) reacts with \(\ce{O2}\) giving a blue flame (Figure \(\PageIndex{1}\)):
\[\ce{S + O_2 \rightarrow SO_2} \nonumber \]
\(\ce{SO2}\) is produced whenever a metal sulfide is oxidized. It is recovered and oxidized further to give \(\mathrm{SO_3}\), for production of \(\mathrm{H_2SO_4}\). \(\mathrm{SO_2}\) reacts with \(\mathrm{H_2S}\) to form \(\mathrm{H_2O}\) and \(\ce{S}\).
\[\mathrm{2 SO_2 + O_2 \rightleftharpoons 2 SO_3} \nonumber \]
\[\mathrm{SO_3 + H_2O \rightleftharpoons H_2SO_4} \;\;(\text{a valuable commodity}) \nonumber \]
\[\mathrm{SO_3 + H_2SO_4 \rightleftharpoons H_2S_2O_7} \;\;\; (\text{pyrosulfuric acid}) \nonumber \]
Sulfur reacts with sulfite ions in solution to form thiosulfate,
\[\ce{S + SO_3^{2-} -> S_2O_3^{2-}} \nonumber \]
but the reaction is reversed in an acidic solution.
Oxides
There are many different stable sulfur oxides, but the two that are commonly found are sulfur dioxide and sulfur trioxide. Sulfur dioxide is a commonly found oxide of sulfur. It is a colorless, pungent, and nonflammable gas. It has a density of 2.8 kg/m3 and a melting point of -72.5 oC. Because organic materials are more soluble in \(SO_2\) than in water, the liquid form is a good solvent. \(SO_2\) is primarily used to produce \(SO_3\). The direct combustion of sulfur and the roasting of metal sulfides yield \(SO_2\) via the contact process:
\[\underbrace{S(s) + O_2(g) \rightarrow SO_2(g)}_{\text{Direct combustion}} \nonumber \]
\[\underbrace{2 ZnS(s) + 3 O_2(g) \rightarrow 2 ZnO(s) + 2 SO_2(g)}_{\text{Roasting of metal sulfides}} \nonumber \]
Sulfur trioxide is another one of the commonly found oxides of sulfur. It is a colorless liquid with a melting point of 16.9 oC and a density of kg/m3. \(SO_3\) is used to produce sulfuric acid. \(SO_2\) is used in the synthesis of \(SO_3\):
\[\underbrace{2 SO_2 (g) + O_2(g) \rightleftharpoons 2 SO_3(g)}_{\text{Exothermic, reversible reaction}} \nonumber \]
This reaction needs a catalyst to be completed in a reasonable amount of time with \(V_2O_5\) being the catalyst most commonly used.
Hydrogen Sulfide H2S
- Hydrogen sulfide, \(\ce{H2S}\), is a diprotic acid. The equilibria below, \[\mathrm{H_2S \rightleftharpoons HS^- + H^+} \nonumber \] \[\mathrm{HS^- \rightleftharpoons S^{2-} + H^+} \nonumber \] have been discussed in connection with Polyprotic Acids.
Other Sulfur-containing Compounds
Perhaps the most significant compound of sulfur used in modern industrialized societies is sulfuric acid (\(H_2SO_4\)). Sulfur dioxide (\(SO_2\)) finds practical applications in bleaching and refrigeration but it is also a nuisance gas resulting from the burning of sulfurous coals. Sulfur dioxide gas then reacts with the water vapor in the air to produce a weak acid, sulfurous acid (\(H_2SO_3\)), which contributes to the acid rain problem.
- Sulfuric acid, H2SO4, is produced by reacting \(SO_3\) with water. However, this often leads to pollution problems. SO3(g) is reacted with 98% H2SO4 in towers full of ceramic material to produce H2S2O7 or oleum. Water is circulated in the tower to maintain the correct concentration and the acid is diluted with water at the end in order to produce the correct concentration. Pure sulfuric acid has no color and odor, and it is an oily, hygroscopic liquid. However, sulfuric acid vapor produces heavy, white smoke and a suffocating odor.
- Dilute sulfuric acid, H2SO4(aq), reacts with metals and acts as a strong acid in common chemical reactions. It is used to produce H2(g) and liberate CO2(g) and can neutralize strong bases.
- Concentrated sulfuric acid, H2SO4 (conc.), has a strong affinity for water. In some cases, it removes H and O atoms. Concentrated sulfuric acid is also a good oxidizing agent and reacts with some metals.
\[C_{12}H_{22}O_{11}(s) \rightarrow 12 C(s) + 11 H_2O(l) \nonumber \]
(Concentrated sulfuric acid used in forward reaction to remove H and O atoms.)
- as a strong acid for making \(\ce{HCl}\) and \(\mathrm{HNO_3}\).
- as an oxidizing agent for metals.
- as a dehydrating agent.
- for manufacture of fertilizer and other commodities.
- Sulfurous acid (H2SO3) is produced when \(SO_2\)(g) reacts with water. It cannot be isolated in its pure form; however, it forms salts as sulfites. Sulfites can act as both reducing agents and oxidizing agents.
O2(g) + 2 SO32-(aq) \(\rightarrow\) 2 SO42- (aq) (Reducing agent)
2 H2S(g) + 2 H+(aq) + SO32-(aq) \(\rightarrow\) 3 H2O(l) + 3 S(s) (Oxidizing agent)
H2SO3 is a diprotic acid that acts as a weak acid in both steps, and H2SO4 is also a diprotic acid but acts as a strong acid in the first step and a weak acid in the second step. Acids like NaHSO3 and NaHSO4 are called acid salts because they are the product of the first step of these diprotic acids.
Boiling elemental sulfur in a solution of sodium sulfite yields thiosulfate. Not only are thiosulfates important in photographic processing, but they are also common analytical reagents used with iodine (like in the following two reactions).
\[2 Cu^{2+}_{(aq)} + 5 I^-_{(aq)} \rightarrow 2 CuI_{(s)} + I^-_{3(aq)} \nonumber \]
\[I^-_{3(aq)} + 2 S_2O^{2-}_{3(aq)} \rightarrow 3 I^-_{(aq)} + S_4O^{2-}_{6(aq)} \nonumber \]
with excess triiodide ion titrated with Na2S2O3(aq).
Other than sulfuric acid, perhaps the most familiar compound of sulfur in the chemistry lab is the foul-smelling hydrogen sulfide gas, \(H_2S\), which smells like rotten eggs.
- Sulfur halides are compounds formed between sulfur and the halogens. Common compounds include SF2, S2F2, SF4, and SF6. While SF4 is a powerful fluorinating agent, SF6 is a colorless, odorless, unreactive gas. Compounds formed by sulfur and chloride include S2Cl2, SCl4, and SCl2. SCl2 is a red, bad-smelling liquid that is utilized to produce mustard gas (\( S(CH_2CH_2Cl)_2\)).
\[SCl_2 + 2CH_2CH_2 \rightarrow S(CH_2CH_2Cl)_2 \nonumber \]
Production -The Frasch Process
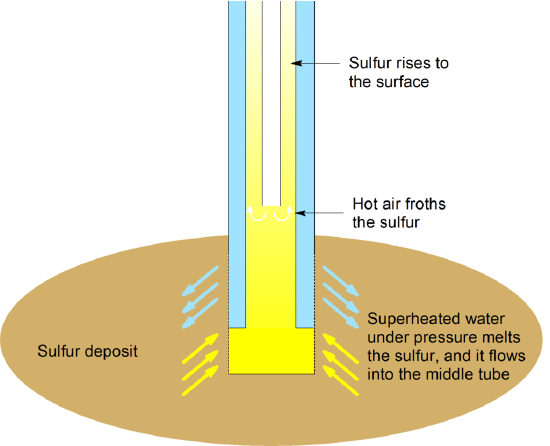
Sulfur can be mined by the Frasch process. This process has made sulfur a high purity (up to 99.9 percent pure) chemical commodity in large quantities. Most sulfur-containing minerals are metal sulfides, and the best known is perhaps pyrite (\(\mathrm{FeS_2}\), known as fool's gold because of its golden color). The most common sulfate-containing mineral is gypsum, \(\mathrm{CaSO_4 \cdot 2H_2O}\), also known as plaster of Paris.
The Frasch process is based on the fact that sulfur has a comparatively low melting point. The process forces (99.5% pure) sulfur out by using hot water and air. In this process, superheated water is forced down the outermost of three concentric pipes. Compressed air is pumped down the center tube, and a mixture of elemental sulfur, hot water, and air comes up the middle pipe. Sulfur is melted with superheated water (at 170 °C under high pressure) and forced to the surface of the earth as a slurry.
Sulfur is mostly used for the production of sulfuric acid, \(\ce{H2SO4}\). Most sulfur mined by the Frasch process is used in industry for the manufacture of sulfuric acid. Sulfuric acid, the most abundantly produced chemical in the United States, is manufactured by the contact process. Most (about 70%) of the sulfuric acid produced in the world is used in the fertilizer industry. Sulfuric acid can act as a strong acid, a dehydrating agent, and an oxidizing agent. Its applications use these properties. Sulfur is an essential element of life in sulfur-containing proteins.
Applications
Sulfur has many practical applications. As a fungicide, sulfur is used to counteract apple scab in organically farmed apple production. Other crops that utilize sulfur fungicides include grapes, strawberries, and many vegetables. In general, sulfur is effective against mildew diseases and black spot. Sulfur can also be used as an organic insecticide. Sulfites are frequently used to bleach paper and preserve dried fruit.
The vulcanization of rubber includes the use of sulfur as well. Cellophane and rayon are produced with carbon disulfide, a product of sulfur and methane. Sulfur compounds can also be found in detergents, acne treatments, and agrichemicals. Magnesium sulfate (epsom salt) has many uses, ranging from bath additives to exfoliants. Sulfur is being increasingly used as a fertilizer as well. Because standard sulfur is hydrophobic, it is covered with a surfactant by bacteria before oxidation can occur. Sulfur is therefore a slow-release fertilizer. Lastly, sulfur functions as a light-generating medium in sulfur lamps.
Concentrated sulfuric acid was once one of the most produced chemicals in the United States; the majority of the H2SO4 that is now produced is used in fertilizer. It is also used in oil refining, production of titanium dioxide, and in emergency power supplies and car batteries. The mineral gypsum, or calcium sulfate dihydrate, is used in making plaster of Paris. Over one million tons of aluminum sulfate is produced each year in the United States by reacting H2SO4 and Al2O3. This compound is important in water purification. Copper sulfate is used in electroplating. Sulfites are used in the paper making industry because they produce a substance that coats the cellulose in the wood and frees the fibers of the wood for treatment.
Emissions and the Environment
Particles, SO2(g), and H2SO4 mist are the components of industrial smog. Because power plants burn coal or high-sulfur fuel oils, SO2(g) is released into the air. When catalyzed on the surfaces of airborne particles, SO2 can be oxidized to SO3. A reaction with NO2 works as well as shown in the following reaction:
\[ SO_{2(g)} + NO_{2(g)} \rightarrow SO_{3(g)} + NO_{(g)} \nonumber \]
H2SO4 mist is then produced after SO3 reacts with water vapor in the air. If H2SO4 reacts with airborne NH3, (NH4)2SO4 is produced. When SO2(g) and H2SO4 reach levels that exceed 0.10 ppm, they are potentially harmful. By removing sulfur from fuels and controlling emissions, acid rain and industrial smog can be kept under control. Processes like fluidized bed combustion have been presented to remove SO2 from smokestack gases.
Outside Links
- Dhawale, S.W. "Thiosulfate: An interesting sulfur oxoanion that is useful in both medicine and industry--but is implicated in corrosion." J. Chem. Educ. 1993, 70, 12.
- Lebowitz, Samuel H. "A demonstration working model of the Frasch process for mining sulfur." J. Chem. Educ. 1931, 8, 1630.
- Nagel, Miriam C. "Herman Frasch, sulfur king (PROFILES)." J. Chem. Educ. 1981, 58, 60.
- Riethmiller, Steven. "Charles H. Winston and Confederate Sulfuric Acid." J. Chem. Educ. 1995 72 575.
- Sharma, B. D. "Allotropes and polymorphs." J. Chem. Educ. 1987, 64, 404.
- Silverstein, Todd P.; Zhang, Yi. "Sugar Dehydration without Sulfuric Acid: No More Choking Fumes in the Classroom!" J. Chem. Educ. 1998 75 748.
- Tykodi, R. J. "In praise of thiosulfate." J. Chem. Educ. 1990, 67, 146.
- Thomas Jefferson National Accelerator Facility - Office of Science Education."It's Elemental-The Element Sulfur." Jefferson Lab.
- Sulfur's Electron Shell
References
- Petrucci, Ralph H. General Chemistry, Principles & Modern Applications. Macmillan Publishing Company, Ninth Edition. Page 930-937.Karchmer, J.H.. The Analytical Chemistry of Sulfur and its Compounds. New York: John Wiley & Sons, Inc., 1970.
Problems
- Draw a diagram that summarizes the allotropy of sulfur. Use symbols, arrows, and numbers.
- Direct combustion of sulfur is the only method for producing SO2(g). True or False.
- Sulfites are not oxidizing agents. They are good reducing agents. True or False.
- Give the reaction for the production of sulfur trioxide.
- Choose the incorrect statement.
- Sulfur produces cellophane and rayon.
- Standard sulfur is hydrophobic.
- SO2 can oxidize to SO3
- Sulfur influences the development of acid rain and industrial smog.
- All of the above are correct.
- Which reaction is responsible for the destruction of limestone and marble statues and buildings?
- \(\ce{CaCO3 \rightarrow CaO + CO2}\)
- \(\ce{SO2 + H2O \rightarrow H2SO3}\)
- \(\ce{BaO + CO2 \rightarrow BaCO3 \rightarrow BaSO3}\) upon reaction with \(\ce{SO2}\)
- \(\ce{CaCO3 + H2O \rightarrow Ca(OH)2 + CO2}\)
- \(\ce{CaCO3 + SO2 \rightarrow CaSO3 + CO2 \rightarrow CaSO4}\) upon oxidation
- Give the formula of thiosulfate ion.
- What is the oxidation state of \(\ce{S}\) in \(\ce{SF6}\), \(\ce{H2SO4}\), \(\ce{NaHSO4}\), \(\ce{SO4^2-}\), and \(\ce{SO3}\)?
- What is the phase of sulfur at 298 K? Enter the type of crystals.
- Give the name of the process by which sulfur is forced out of the ground using hot water and air.
Solutions
- The diagram may be drawn in any way. However, the symbols (S2), (S4), (S6), (S?), and (S8(g)) must be included. The temperatures should be written next to the arrows.
- False
- False
- \(2 SO_{2(g)} + O_{2(g)} \rightarrow 2 SO_{3(g)}\)
- A
- e.
Consider...
\(\ce{SO2}\) in \(\ce{H2SO3}\) is the acid in acid rain, which attacks \(\ce{CaCO3}\), marble. \(\ce{SO2}\) reduces pigments in organic matter. - \(\ce{S2O3^2-}\)
Consider...
Sulfate is \(\ce{SO4^2-}\); replacement of an \(\ce{O}\) by an \(\ce{S}\) gives thiosulfate \(\ce{S2O3^2-}\). The two \(\ce{S}\) in \(\ce{S2O3^2-}\) have different oxidation states: one is +6, the other is (-2), average +2. - 6
Consider...
Oxidation state for \(\ce{S}\) in \(\ce{H2SO3}\), \(\ce{SO3^2-}\), \(\ce{SO2}\), etc. is 4. The oxidation state of \(\ce{S}\) is the same for all in the list. - rhombic sulfur
Consider...
The term rhombic describes a type of crystal. Monoclinic sulfur is meta stable at 298 K. - Frasch process
Consider...
The Frasch process is used to mine elemental sulfur.
Contributors and Attributions
- StackExchange
Chung (Peter) Chieh (Professor Emeritus, Chemistry @ University of Waterloo)

