Physical Properties of the Group 17 Elements
- Page ID
- 582
This page discusses the trends in some atomic and physical properties of the Group 17 elements (the halogens): fluorine, chlorine, bromine and iodine. Sections below describe the trends in atomic radius, electronegativity, electron affinity, melting and boiling points, and solubility. There is also a section on the bond enthalpies (and strengths) of halogen-halogen bonds (for example, the Cl-Cl bond) and of hydrogen-halogen bonds (e.g., the H-Cl bond).
Trends in Atomic Radius
You can see that the atomic radius increases as you go down the group.
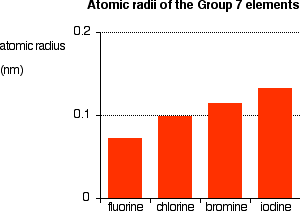
The radius of an atom is governed by
- Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. It is usually measured on the Pauling scale, on which the most electronegative element (fluorine) is given an electronegativity of 4.0.
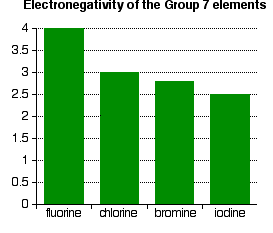
As shown in the figure above, electronegativity decreases from fluorine to iodine; the atoms become less effective at attracting bonding pairs of electrons as they grow larger. This can be visualized using dots-and-crosses diagrams for hydrogen fluoride and hydrogen chloride.
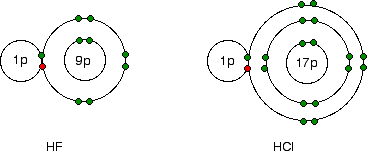
The bonding electrons between the hydrogen and the halogen experience the same net charge of +7 from either the fluorine or the chlorine. However, in the chlorine case, the nucleus is further away from the bonding pair. Therefore, electrons are not as strongly attracted to the chlorine nucleus as they are to the fluorine nucleus.
The stronger attraction to the closer fluorine nucleus makes fluorine more electronegative.
Summarizing the trend down the group
As the halogen atoms get larger, any bonding pair is farther and farther away from the halogen nucleus, and so is less strongly attracted towards it. Hence, the elements become less electronegative as you go down the group.
Contributors and Attributions
Jim Clark (Chemguide.co.uk)


