Chemistry of Hydrogen (Z=1)
- Page ID
- 590
Hydrogen is a colorless, odorless and tasteless gas that is the most abundant element in the known universe. It is also the lightest (in terms of atomic mass) and the simplest, having only one proton and one electron (and no neutrons in its most common isotope). It is all around us. It is a component of water (H2O), fats, petroleum, table sugar (C6H12O6), ammonia (NH3), and hydrogen peroxide (H2O2)—things essential to life, as we know it.
Hydrogen Facts
- Atomic Number: 1
- Atomic Symbol: H
- Atomic Weight: 1.0079
- Electronic Configuration: 1s1
- Oxidation States: 1, -1
- Atomic Radius: 78 pm
- Melting Point: -259.34°C
- Boiling Point: -252.87° C
- Elemental Classification: Non-Metal
- At Room Temperature: Colorless & Odorless Diatomic Gas
History of Hydrogen
Hydrogen comes from Greek meaning “water producer” (“hydro” =water and “gennao”=to make). First isolated and identified as an element by Cavendish in 1766, hydrogen was believed to be many different things. Cavendish himself thought that it was "inflammable air from metals", owing to its production by the action of acids on metals. Before that, Robert Boyle and Paracelsus both used reactions of iron and acids to produce hydrogen gas and Antoine Lavoisier gave hydrogen its name because it produced water when ignited in air. Others thought it was pure phlogiston because of its flammability. Hydrogen is among the ten most abundant elements on the planet, but very little is found in elemental form due to its low density and reactivity. Much of the terrestrial hydrogen is locked up in water molecules and organic compounds like hydrocarbons.
Properties of Hydrogen
Hydrogen is a nonmetal and is placed above group in the periodic table because it has ns1 electron configuration like the alkali metals. However, it varies greatly from the alkali metals as it forms cations (H+) more reluctantly than the other alkali metals. Hydrogen‘s ionization energy is 1312 kJ/mol, while lithium (the alkali metal with the highest ionization energy) has an ionization energy of 520 kJ/mol.
Because hydrogen is a nonmetal and forms H- (hydride anions), it is sometimes placed above the halogens in the periodic table. Hydrogen also forms H2 dihydrogen like halogens. However, hydrogen is very different from the halogens. Hydrogen has a much smaller electron affinity than the halogens.
H2 dihydrogen or molecular hydrogen is non-polar with two electrons. There are weak attractive forces between H2 molecules, resulting in low boiling and melting points. However, H2 has very strong intramolecular forces; H2 reactions are generally slow at room temperature due to strong H—H bond. H2 is easily activated by heat, irradiation, or catalysis. Activated hydrogen gas reacts very quickly and exothermically with many substances.
Hydrogen also has an ability to form covalent bonds with a large variety of substances. Because it makes strong O—H bonds, it is a good reducing agent for metal oxides. Example: CuO(s) + H2(g) → Cu(s) + H2O(g) H2(g) passes over CuO(s) to reduce the Cu2+ to Cu(s), while getting oxidized itself.
Reactions of Hydrogen
Hydrogen's low ionization energy makes it act like an alkali metal:
\[H_{(g)} \rightarrow H^+_{(g)} + e^- \nonumber \]
However, it half-filled valence shell (with a \(1s^1\) configuration) with one \(e^-\) also causes hydrogen to act like a halogen non-metal to gain noble gas configuration by adding an additional electron
\[H_{(g)} + e^- \rightarrow H^-_{(g)} \nonumber \]
Reactions of Hydrogen with Active Metals
Hydrogen accepts e- from an active metal to form ionic hydrides like LiH. By forming an ion with -1 charge, the hydrogen behaves like a halogen.
Group 1 metals
\[2M_{(s)}+H_{2(g)} \rightarrow 2MH_{(s)} \nonumber \]
with \(M\) representing Group 1 Alkali metals
Examples:
- \(2K_{(s)}+H_{2(g)} \rightarrow 2KH_{(s)}\)
- \(2K_{(s)}+Cl_{2(g)} \rightarrow 2KCl_{(s)}\)
Group 2 metals
\[M_{(s)}+H_{2(g)} \rightarrow MH_{2(s)} \nonumber \]
with \(M\) representing Group 2 Alkaline Earth metals
Example:
- \(Ca_{(s)}+H_{2(g)} \rightarrow CaH_{2(s)}\)
- \(Ca_{(s)}+Cl_{2(g)} \rightarrow CaCl_{2(s)}\)
Reactions of Hydrogen with Nonmetals
Unlike metals forming ionic bonds with nonmetals, hydrogen forms polar covalent bonds. Despite being electropositive like the active metals that form ionic bonds with nonmetals, hydrogen is much less electropositive than the active metals, and forms covalent bonds.
Hydrogen + Halogen → Hydrogen Halide
\[H_{2(g)}+ Cl_{2(g)} \rightarrow HCl_{(g)} \nonumber \]
Hydrogen gas reacting with oxygen to produce water and a large amount of heat: Hydrogen + Oxygen → Water
\[(H_{2(g)}+O_{2(g)} \rightarrow H_2O_{(g)} \nonumber \]
Reactions with Transition Metals
Reactions of hydrogen with Transition metals (Group 3-12) form metallic hydrides. There is no fixed ratio of hydrogen atom to metal because the hydrogen atoms fill holes between metal atoms in the crystalline structure.
Uses & Application
The vast majority of hydrogen produced industrially today is made either from treatment of methane gas with steam or in the production of "water gas" from the reaction of coal with steam. Most of this hydrogen is used in the Haber process to manufacture ammonia.
Hydrogen is also used for hydrogenation vegetable oils, turning them into margarine and shortening, and some is used for liquid rocket fuel. Liquid hydrogen (combined with liquid oxygen) is a major component of rocket fuel (as mentioned above combination of hydrogen and oxygen relapses a huge amount of energy). Because hydrogen is a good reducing agent, it is used to produce metals like iron, copper, nickel, and cobalt from their ores.
Because one cubic feet of hydrogen can lift about 0.07 lbs, hydrogen lifted airships or Zeppelins became very common in the early 1900s.However, the use of hydrogen for this purpose was largely discontinued around World War II after the explosion of The Hindenburg; this prompted greater use of inert helium, rather than flammable hydrogen for air travel.
Video Showing the explosion of The Hindenburg. (Video from Youtube)
Recently, due to the fear of fossil fuels running out, extensive research is being done on hydrogen as a source of energy.Because of their moderately high energy densities liquid hydrogen and compressed hydrogen gas are possible fuels for the future.A huge advantage in using them is that their combustion only produces water (it burns “clean”). However, it is very costly, and not economically feasible with current technology.
Combustion of fuel produces energy that can be converted into electrical energy when energy in the steam turns a turbine to drive a generator. However, this is not very efficient because a great deal of energy is lost as heat. The production of electricity using voltaic cell can yield more electricity (a form of usable energy). Voltaic cells that transform chemical energy in fuels (like H2 and CH4) are called fuel cells. These are not self-contained and so are not considered batteries. The hydrogen cell is a type of fuel cell involving the reaction between H2(g) with O2(g) to form liquid water; this cell is twice as efficient as the best internal combustion engine. In the cell (in basic conditions), the oxygen is reduced at the cathode, while the hydrogen is oxidized at the anode.
Reduction: O2(g)+2H2O(l)+4e- → 4OH-(aq)
Oxidation: H2(g) + 2OH-(aq) → 2H2O(l) + 2e-
Overall: 2H2(g) + O2(g) → 2H2O(l)
E°cell= Reduction- Oxidation= E°O2/OH- - E°H2O/H2 = 0.401V – (-0.828V) = +1.23
However, this technology is far from being used in everyday life due to its great costs.
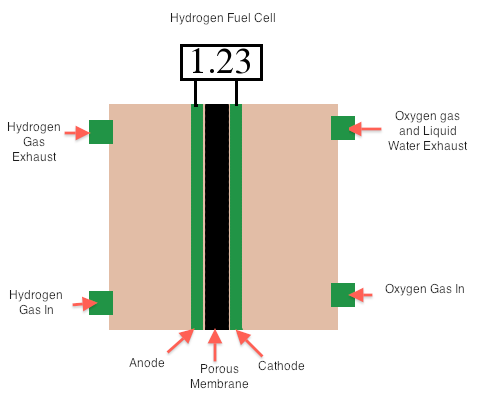
Image of A Hydrogen Fuel Cell. (Image made by Ridhi Sachdev)
Natural Occurrence & Other Sources
Naturally Occurring Hydrogen
Hydrogen is the fuel for reactions of the Sun and other stars (fusion reactions). Hydrogen is the lightest and most abundant element in the universe. About 70%- 75% of the universe is composed of hydrogen by mass. All stars are essentially large masses of hydrogen gas that produce enormous amounts of energy through the fusion of hydrogen atoms at their dense cores. In smaller stars, hydrogen atoms collided and fused to form helium and other light elements like nitrogen and carbon(essential for life). In the larger stars, fusion produces the lighter and heavier elements like calcium, oxygen, and silicon.
On Earth, hydrogen is mostly found in association with oxygen; its most abundant form being water (H2O). Hydrogen is only .9% by mass and 15% by volume abundant on the earth, despite water covering about 70% of the planet. Because hydrogen is so light, there is only 0.5 ppm (parts per million) in the atmosphere, which is a good thing considering it is EXTREMELY flammable.
Other Sources of Hydrogen
Hydrogen gas can be prepared by reacting a dilute strong acid like hydrochloric acids with an active metal. The metal becomes oxides, while the H+ (from the acid) is reduced to hydrogen gas. This method is only practical for producing small amounts of hydrogen in the lab, but is much too costly for industrial production:
\[Zn_{(s)} + 2H^+_{(aq)} \rightarrow Zn^{2+}_{(aq)} + H_{2(g)} \nonumber \]
The purest form of H2(g) can come from electrolysis of H2O(l), the most common hydrogen compound on this plant. This method is also not commercially viable because it requires a significant amount of energy (\(\Delta H = 572 \;kJ\)):
\[2H_2O_{(l)} \rightarrow 2H_{2(g)} + O_{2(g)} \nonumber \]
\(H_2O\) is the most abundant form of hydrogen on the planet, so it seems logical to try to extract hydrogen from water without electrolysis of water. To do so, we must reduce the hydrogen with +1 oxidation state to hydrogen with 0 oxidation state (in hydrogen gas). Three commonly used reducing agents are carbon (in coke or coal), carbon monoxide, and methane. These react with water vapor form H2(g):
\[C_{(s)} + 2H_2O_{(g)} \rightarrow CO(g) + H_{2(g)} \nonumber \]
\[CO_{(g)} + 2H_2O_{(g)} \rightarrow CO2 + H_{2(g)} \nonumber \]
Reforming of Methane:
\[CH_{4(g)} + H_2O_{(g)} \rightarrow CO(g) + 3H_{2(g)} \nonumber \]
These three methods are most industrially feasible (cost effective) methods of producing H2(g).
Isotopes
There are two important isotopes of hydrogen. Deuterium (2H) has an abundance of 0.015% of terrestrial hydrogen and the nucleus of the isotope contains one neutron.
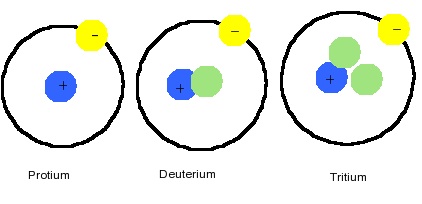
- Protium (1H) is the most common isotope, consisting of 99.98% of naturally occurring hydrogen. It is a nucleus containing a single proton.
- Deuterium (2H) is another an isotope containing a proton and neutron, consisting of only 0.0156% of the naturally occurring hydrogen. Commonly indicated with symbol D and sometimes called heavy hydrogen, deuterium is separated by the fractional distillation of liquid hydrogen but it can also be produced by the prolonged electrolysis of ordinary water. Approximately 100,000 gallons of water will produce a single gallon of D2O, "heavy water". This special kind of water has a higher density, melting point, and boiling point than regular water and used as a moderator in some fission power reactors. Deuterium fuel is used in experimental fusion reactors. Replacing protium with deuterium has important uses for exploring reaction mechanisms via the kinetic isotope effect.
- Tritium (3H) contains two neutrons in its nucleus and is radioactive with a 12.3-year half-life, which is continuously formed in the upper atmosphere due to cosmic rays. It is can also be made in a lab from Lithium-6 in a nuclear reactor. Tritium is also used in hydrogen bombs. It is very rare (about 1 in every 1,018 atoms) and is formed in the environment by cosmic ray bombardment. Most tritium is manufactured by bombarding Li with neutrons. Tritium is used in thermonuclear weapons and experimental fusion reactors.
References
- Shultz, M., Kelly, M., Paritsky, L., Wagner, J. A Theme-Based Course: Hydrogen as the Fuel of the Future. Journal of Chemical Education 2009 86 (9), 105.
- Rigden, John. Hydrogen: The Essential Element. The President and Fellows of Harvard College. 2003.
- Banks, Alton. Hydrogen. Journal of Chemical Education 1989 66 (10), 801.
- Petrucci, Ralph H. General Chemistry. 9th ed. Upper Saddle River: Prentice Hall, 2007. Print
- Sadava, Heller, Orians, Purves, Hillis. Life The Science of Biology. 8th ed. Sunderland, MA: W.H. Freeman, 2008.
- Dinga, G. Hydrogen:The ultimate fuel and energy carrier. Journal of Chemical Education 1988 65 (8), 688.
Outside Links
Problems
- Write the reaction of Na(s) with H2(g).
- What is the name of the radioactive isotope of hydrogen?
- What characteristics of alkali metals does hydrogen display?
- What characteristics of halogens does hydrogen display?
- How does the electronegativity of hydrogen compare to that of the halogens?
- What is the electron configuration of a neutral hydrogen atom.
Answers
- 2Na(s) + H2(g)→ 2NaH(s)
- Tritium
- Hydrogen is placed above group in the periodic table because it has ns1 electron configuration like the alkali metals. However, it varies greatly from the alkali metals as it forms cations (H+) more reluctantly than the other alkali metals. Hydrogen‘s ionization energy is 1312 kJ/mol, while lithium (the alkali metal with the highest ionization energy) has an ionization energy of 520 kJ/mol.
- Because hydrogen is a nonmetal and forms H- (hydride anions), it is sometimes placed above the halogens in the periodic table. Hydrogen also forms H2 dihydrogen like halogens. However, hydrogen is very different from the halogens. Hydrogen has a much smaller electron affinity than the halogens.
- Hydrogen is less electronegative than the halogens.
- 1s1
Contributors and Attributions
- Ridhi Sachdev (UC Davis)
Stephen R. Marsden

