Reactions of Acyl Chlorides Involving Oxygen Compounds
- Page ID
- 3933
This page discusses the reactions of acyl chlorides (acid chlorides) with water, alcohols and phenol. These reactions are considered together because their chemistry is similar.
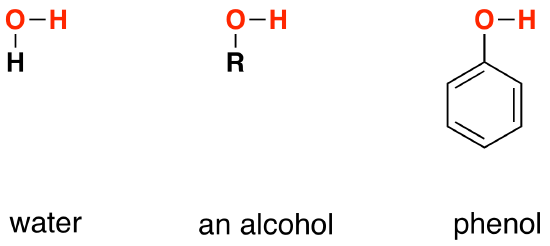
Comparing the structures of water, ethanol and phenol
Each of these substances contains an -OH group. In water, this is attached to a hydrogen atom. In an alcohol, it is attached to an alkyl group, indicated in the diagrams below as "R". In phenol, it is attached to a benzene ring (phenol is C6H5OH).
General Reactions with acyl chlorides
Consider ethanoyl chloride as a typical example of the acyl chlorides. Taking a general case of a reaction between ethanoyl chloride and a compound X-O-H (where X is hydrogen, or an alkyl group or a benzene ring):

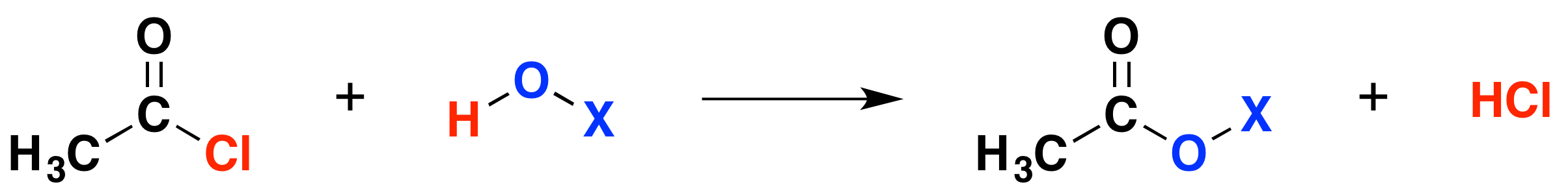
In each case, hydrogen chloride gas is produced, the hydrogen coming from the -OH group, and the chlorine from the ethanoyl chloride. The remaining species are joined together.
The reaction with water
Modifying the general equation above, ethanoic acid is produced together with hydrogen chloride gas.


This is more often written as follows:
\[ 3CH_3COCl + H_2O \rightarrow CH_3COOH + HCl\]
Adding an acyl chloride to water produces the corresponding carboxylic acid together with steamy acidic fumes of hydrogen chloride. The reaction is usually extremely vigorous at room temperature.
The reaction with alcohols
The general equation for any alcohol reacting with ethanoyl chloride is the following:

The organic product in this case is an ester. For example, ethanol produces the ester ethyl ethanoate:
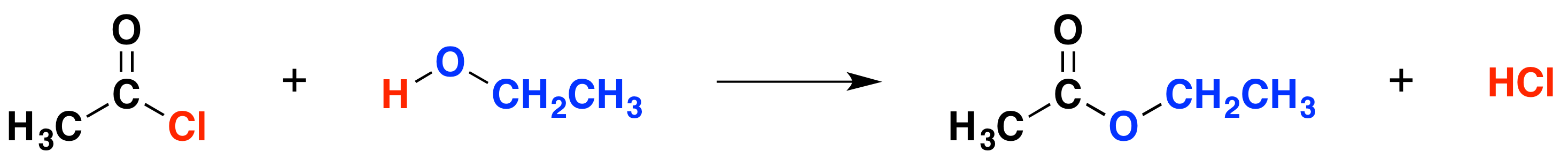
The equation is more commonly written as follows:
\[ CH_3COCl + CH_3CH_2OH \rightarrow CH_3COOCH_2CH_3 + HCl\]
The reaction with phenols
A phenol has an -OH group attached directly to a benzene ring. In the substance normally called "phenol", there is nothing else attached to the ring; this simplest case is considered first. The reaction between phenol and ethanoyl chloride is not quite as vigorous as that between alcohols and ethanoyl chloride; the reactivity of the -OH group is modified by the benzene ring.
Aside from this, the reaction is just the same as with an alcohol:


Written more simply:
\[ CH_3COCl + C_6H_5OH \rightarrow CH_3COOC_6H_5 + HCl\]
Particularly with equation in this second form, it is obvious that another ester is produced, in this case phenyl ethanoate. However, it this product is often drawn a variety of ways that make it appear as a derivative of phenol (which it is).
For example:

This form is useful when concentrating on the reactions of the phenol rather than the acyl chloride. In this form, it is apparent that the hydrogen of the phenol -OH group has been replaced by an acyl group, an alkyl group attached to a carbon-oxygen double bond. The phenol is said to have been acylated or to have undergone acylation. Because of the nature of this particular acyl group, it is also described as ethanoylation. The hydrogen is replaced by an ethanoyl group, CH3CO-.
Using a similar reaction to make aspirin
The reaction with phenol itself is not very important, but aspirin can be made in a similar reaction. Below is shown 2-hydroxybenzoic acid (also known as 2-hydroxybenzenecarboxylic acid). The old name for this compound is salicylic acid. This may be written in either of the following two ways. They are the same structure with the molecule flipped in space.

When this compound reacts with ethanoyl chloride, it is ethanoylated (or acylated, to use the more general term) to give,

This molecule is aspirin.
Contributors
Jim Clark (Chemguide.co.uk)


