Reactions of Acyl Chlorides with Water
- Page ID
- 3794
This page gives you the facts and a simple, uncluttered mechanism for the nucleophilic addition / elimination reaction between acyl chlorides (acid chlorides) and water. Ethanoyl chloride is taken as a typical acyl chloride. Any other acyl chloride will behave in the same way. Simply replace the CH3 group in what follows by anything else you want.
The facts
Ethanoyl chloride reacts instantly with cold water. There is a very exothermic reaction in which a steamy acidic gas is given off (hydrogen chloride) and ethanoic acid is formed.

The mechanism
The first stage (the addition stage of the reaction) involves a nucleophilic attack on the fairly positive carbon atom by one of the lone pairs on the oxygen of a water molecule.
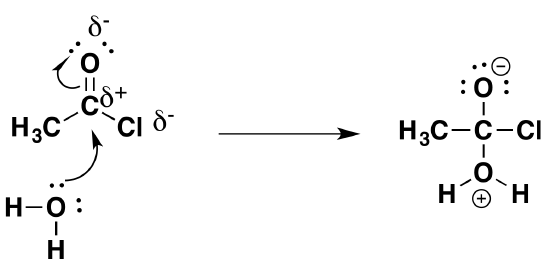
The second stage (the elimination stage) happens in two steps. In the first, the carbon-oxygen double bond reforms and a chloride ion is pushed off.
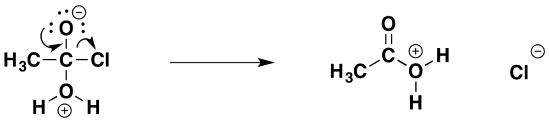
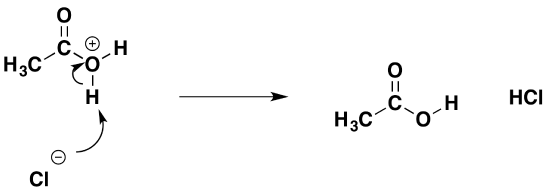
That is followed by removal of a hydrogen ion by the chloride ion to give ethanoic acid and hydrogen chloride.
Contributors
Jim Clark (Chemguide.co.uk)


