Using Acyl Chlorides in Friedel-Crafts Reactions
- Page ID
- 3935
This page discusses the reaction of acyl chlorides (acid chlorides) with benzene in the presence of an aluminum chloride catalyst. This is known as a Friedel-Crafts acylation.
Friedel-Crafts acylation of benzene
Acylation is the term given to substituting an acyl group such as CH3CO- onto another molecule. An acyl group is a hydrocarbon group attached to a carbon-oxygen double bond. The most common example of an acyl group is the ethanoyl group, CH3CO-, and this group is used throughout this article. If benzene is reacted with ethanoyl chloride in the presence of an aluminium chloride catalyst, the equation for the reaction is as follows:
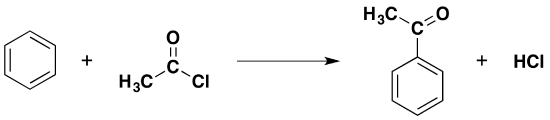

This equation can be simplified as follows:
\[ C_6H_6 + CH_3COCl \rightarrow C_6H_6COCH_3 + HCl\]
In the simplified formula for the product, the phenyl group is usually written on the left side and the alkyl group to the right of the carbon-oxygen double bond. The aluminum chloride is not written into these equations because it acts as a catalyst. It could be included as an AlCl3 over the top of the arrow. The product is called phenylethanone (old name: acetophenone).
Reaction conditions
Ethanoyl chloride is added carefully to a mixture of benzene and solid aluminium chloride cold conditions. Hydrogen chloride gas is given off. When all the ethanoyl chloride has been added, the mixture is heated under reflux at a temperature of 60°C for about 30 minutes to complete the reaction.
The importance of this reaction
Friedel-Crafts acylation is a very effective way of attaching a hydrocarbon-based group to a benzene ring. Although the product is a ketone (a compound containing a carbon-oxygen double bond with a hydrocarbon group either side), it is easily converted into other species. For example, the carbon-oxygen double bond can be reduced to give a secondary alcohol, which in turn can undergo a many other reactions.
Reduction of phenylethanone to produce ethylbenzene
This is known as the Clemmensen reduction, and involves heating the ketone with amalgamated zinc (a mixture of zinc and mercury) and concentrated hydrochloric acid for a long time.
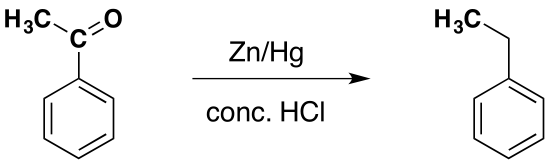
This indirect route is the best way to attach an alkyl group to a benzene ring. It is possible to attach an alkyl group directly to the ring, but it is impossible to limit the reaction to one substitution. An alkyl group attached to the ring makes the ring more reactive than the original benzene, meaning like ethylbenzene (for example) reacts faster than benzene itself.
The result is that several ethyl groups are substituted around the ring, rather than just one. Acyl groups, however, have the opposite effect. Attaching an acyl group to the ring makes the ring so unreactive that a second one cannot be substituted.
Contributors
Jim Clark (Chemguide.co.uk)


