Properties of Alcohols
- Page ID
- 735
Alcohols are some of the most important molecules in organic chemistry. They can be prepared from and converted into many different types of compounds. Alcohols contain the hydroxy functional group (-OH), bonded to a carbon atom of an alkyl or substituted alkyl group. The functional group of an alcohol is the hydroxyl group, –OH. Unlike the alkyl halides, this group has two reactive covalent bonds, the C–O bond and the O–H bond. The electronegativity of oxygen is substantially greater than that of carbon and hydrogen. Consequently, the covalent bonds of this functional group are polarized so that oxygen is electron rich and both carbon and hydrogen are electrophilic, as shown in the figure below.
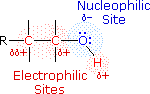
Indeed, the dipolar nature of the O–H bond is such that alcohols are much stronger acids than alkanes (by roughly 1030 times), and nearly that much stronger than ethers. The most reactive site in an alcohol molecule is the hydroxyl group, despite the fact that the O–H bond strength is significantly greater than that of the C–C, C–H and C–O bonds, demonstrating again the difference between thermodynamic and chemical stability.

