Oxidation by Chromic Acid
- Page ID
- 15384
One of the reagents that is commonly used for oxidation in organic chemistry is chromic acid. This reagent is straightforward to use once deciphered. However, there are a vast number of different ways that textbooks (and instructors) show it being used in reactions.
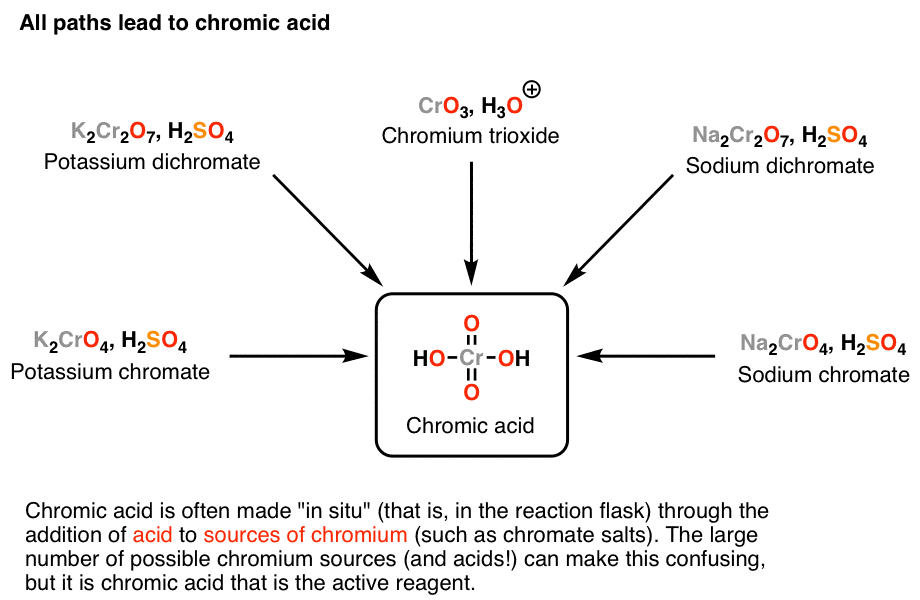
Chromic acid, H2CrO4, is a strong acid and a reagent for oxidizing alcohols to ketones and carboxylic acids. For fairly mundane reasons owing primarily to safety and convenience, chromic acid tends to be made in the reaction vessel as needed (through addition of acid to a source of chromium), rather than being dispensed from a bottle.
And that’s where the trouble begins. Choosing a source of chromium to make H2CrO4 from is a lot like choosing a favorite brand of bottled water. Beyond the packaging, they’re pretty much all the same. Depending on which textbook or instructor you have, however, you might see several different ways to do this, and it can be very confusing.
The key point is that Na2CrO4 (sodium chromate), Na2Cr2O7 (sodium dichromate), K2CrO4(potassium chromate), K2Cr2O7 (potassium dichromate), and CrO3 (chromium trioxide) are all alike in one crucial manner: when they are combined with aqueous acid, each of them forms H2CrO4, and ultimately it’s H2CrO4 which does the important chemistry. Unfortunately I rarely see this point explained in textbooks. I remember this causing some confusion for me when I took the course. The K or Na ions present are just spectators.
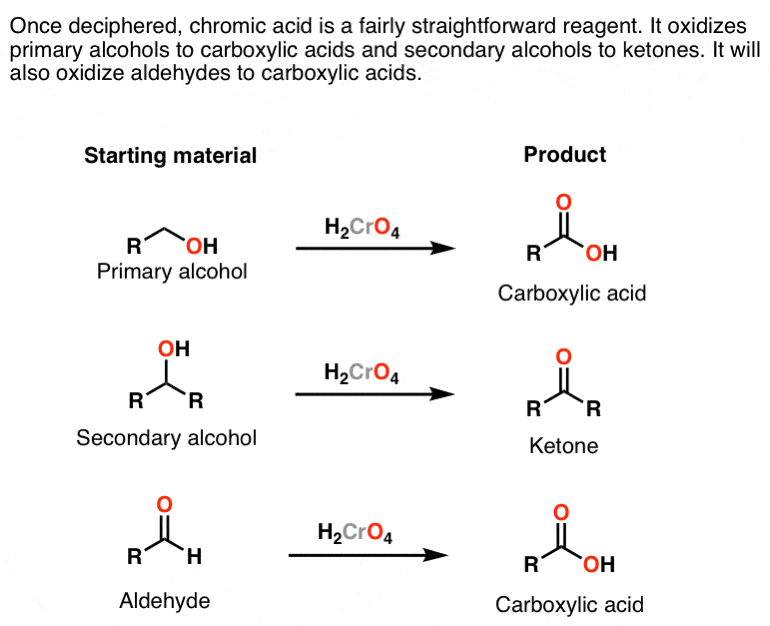
Once H2CrO4 is formed, its reactions are pretty straightforward: it converts primary alcohols (and aldehydes) to carboxylic acids and secondary alcohols to ketones.
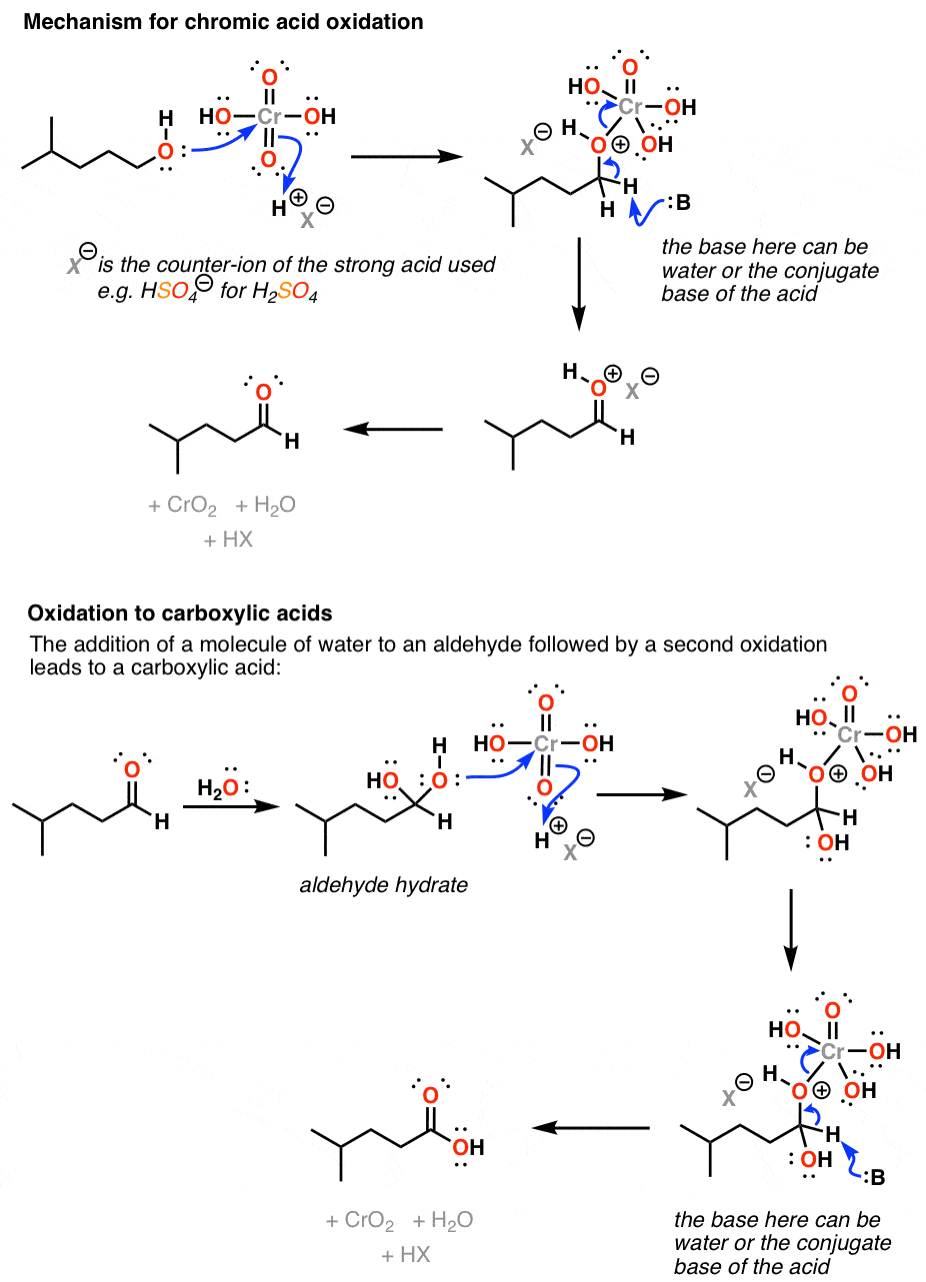
It does this through addition of the alcohol oxygen to chromium, which makes it a good leaving group; a base (water being the most likely culprit) can then remove a proton from the carbon, forming a new π bond and breaking the O-Cr bond.
Due to its high toxicity, chromic acid tends to find very little use in the organic chemistry laboratory outside of undergrad labs. There are far more useful reagents out there for performing these transformations.
Contributors
James Ashenhurst (MasterOrganicChemistry.com)

