Nomenclature of Amides
- Page ID
- 5888
Primary amides
Primary amides are named by changing the name of the acid by dropping the -oic acid or -ic acid endings and adding -amide. The carbonyl carbon is given the #1 location number. It is not necessary to include the location number in the name because it is assumed that the functional group will be on the end of the parent chain.
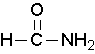
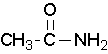
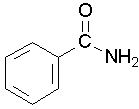
methanamide or formamide (left), ethanamide or acetamide (center) , benzamide (right)
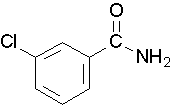
Draw a structure for the following compound: 3-chlorobenzamide.
Solution
Try to name the following compound:
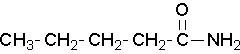
Solution
pentamide
Secondary Amides
Secondary amides are named by using an upper case N to designate that the alkyl group is on the nitrogen atom. Alkyl groups attached to the nitrogen are named as substituents. The letter N is used to indicate they are attached to the nitrogen.
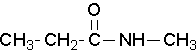
N-methylpropanamide
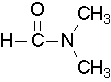
Try to draw a structure for the following compound: N,N-dimethylformamide.
Solution
Try to name the following compound:
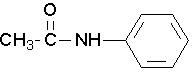
N-phenylethanamide or N-phenylacetamide
Tertiary Amides
Tertiary amides are named in the same way as secondary amides, but with two N's
Try to draw a structure for N,N-dimethylformamide.
Solution

Contributors
Prof. Steven Farmer (Sonoma State University)

