VII. Radical Combination
- Page ID
- 23825
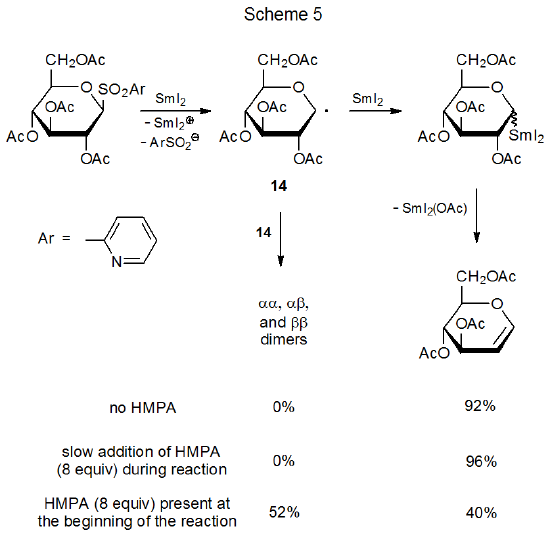
Radical combination involving carbohydrates takes place either by reaction between two carbohydrate radicals or between a carbohydrate and a noncarbohydrate radical (eq 50). Successful radical combination requires that the rates of competing reactions (e.g., hydrogen-atom abstraction and radical addition) be reduced to the point that radicals exist long enough in solution to combine. Under most conditions the lifetimes of typical carbohydrate radicals are too short for two of them to diffuse through solution and react. If conditions are selected to minimize competing reactions, pyranos-1-yl radicals, which are among the most stable carbohydrate radicals, exist in solution long enough to come into contact with each other and thus form dimers, although in low yield (eq 51).39 If conditions are chosen that produce large numbers of radicals in a short period of time, radical concentration can be raised to the point where substantial combination takes place (Scheme 5).40
.png?revision=1&size=bestfit&width=245&height=74)
.png?revision=1&size=bestfit&width=355&height=133)
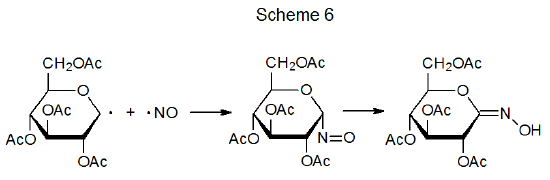
The noncarbohydrate radicals taking part in radical combination have a range of possible structures. The basic requirement in most instances is that the noncarbohydrate radical be sufficiently stable that its concentration in solution builds to the point that it will combine quickly with the more reactive carbohydrate radicals as they are produced. [Radicals with considerable stability are described as being either persistent or stable (Chapter 2, Section I). The presence of such radicals provides the basis for the “persistent radical effect” discussed in Chapter 3 (Section II.B.1.c.).] Noncarbohydrate participants in radical combination range from stable compounds, such as nitric oxide (Scheme 6),41 to resonance-stabilized radicals, such as the 2‑pyridylthiyl radical (eq 52).23 Electrolysis is different from most reactions because it can produce locally high enough concentrations of radicals to allow even reactive ones to combine (eq 53).42
.png?revision=1&size=bestfit&width=430&height=81)
.png?revision=1&size=bestfit&width=415&height=132)
Some electron-transfer reactions between carbohydrate radicals and transition-metal complexes have a similarity to radical combination. In the reaction shown in eq 54, for instance, a change in oxidation states accompanies the combination between the carbohydrate radical and the cobalt complex.27
.png?revision=1&size=bestfit&width=420&height=111)

