VI. Electron Transfer
- Page ID
- 23823
A. Reactions of Carbohydrate Radicals
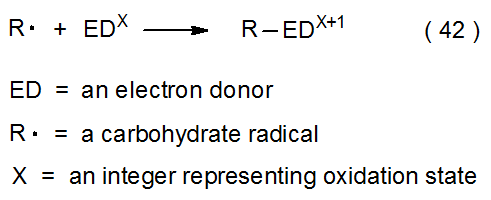
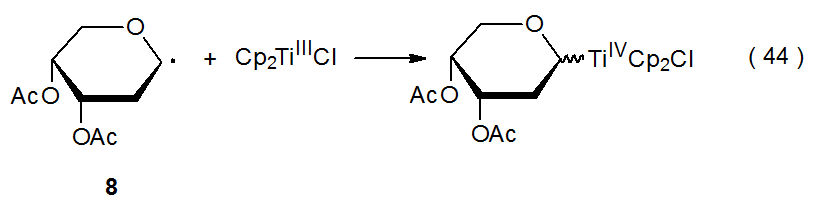
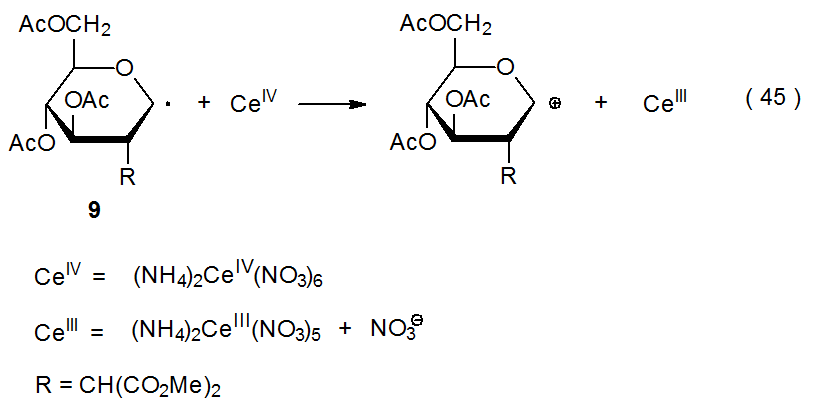
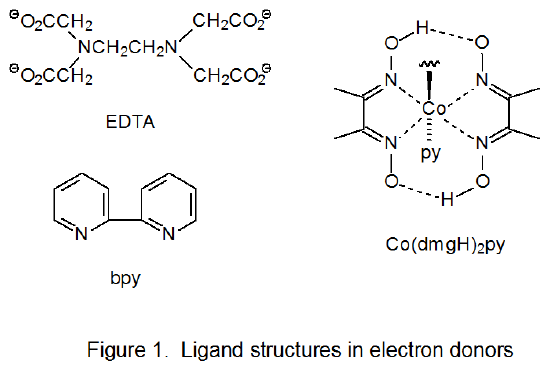
Transition-metal complexes can act as electron transfer agents when reacting with carbohydrate radicals (eq 42 and 43). In the reaction shown in eq 44, titanium donates an electron during formation of the carbon-titanium bond between titanocene(III) chloride (Cp2TiCl) and the pyranos-l‑yl radical 8.31,32 In the reaction shown in eq 45, ammonium cerium nitrate [(NH4)2Ce(NO3)6] accepts an electron from the pyranos-1-yl radical 9 to convert it into the corresponding cation.33,34 Other compounds that serve as electron donors in reactions with carbohydrate radicals are SmI2, Cr(EDTA)2-, [Ru(bpy)3]2+, and Co(dmgH)2py. (The structures of the ligands in these compounds are pictured in Figure 1). In addition to (NH4)2Ce(NO3)6, Mn(OAc)3 also acts as an electron acceptor in reactions with carbohydrate radicals.
.png?revision=1&size=bestfit&width=245&height=100)
.png?revision=1&size=bestfit&width=245&height=100)
.png?revision=1&size=bestfit&width=410&height=105)
.png?revision=1&size=bestfit&width=410&height=208)
B. Formation of Carbohydrate Radicals
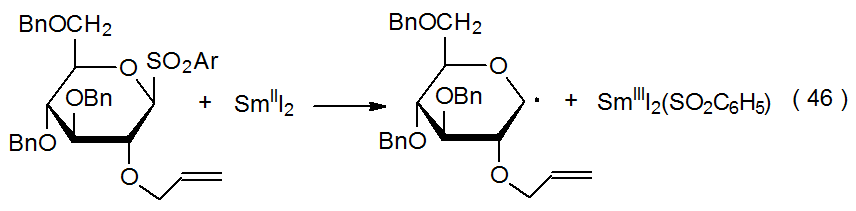
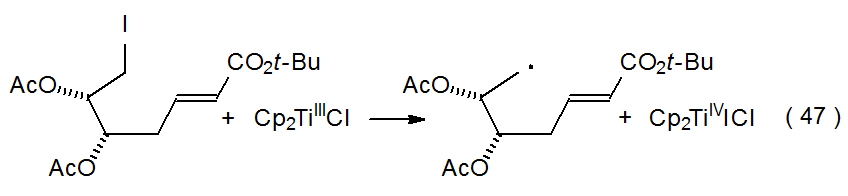
When an electron is transferred to a carbohydrate chloride, bromide, iodide, or sulfone, the resulting radical anion reacts to form a carbohydrate radical (Scheme 4). The two electron donors participating in most of these reactions are SmI2 (eq 46)35 and Cp2TiCl (eq 47),36 but other transition-metal complexes [e.g., Cr(EDTA)2-] are able to function in this capacity. Although the radical anion 10 is pictured in Scheme 4 as a discrete intermediate, in some instances cleavage of the RX bond may be simultaneous with electron transfer.
.png?revision=1&size=bestfit&width=430&height=106)
.png?revision=1&size=bestfit&width=425&height=98)
Solvated electrons, which are more reactive as electron donors than transition-metal complexes, combine with esterified carbohydrates to produce radical anions. These radical anions then expel carboxylate anions to form carbohydrate radicals (eq 48).37
.png?revision=1&size=bestfit&width=415&height=119)
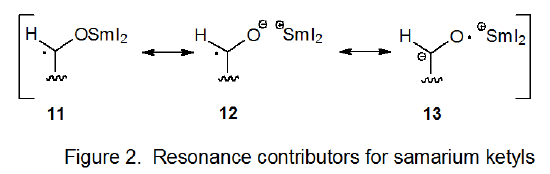
Reaction of an aldehyde or ketone with samarium(II) iodide produces a samarium ketyl (eq 49),38 an intermediate considered to be a hybrid of structures 11-13 (Figure 2). These ketyls exhibit reactivity characteristic of carbon-centered radicals.
.png?revision=1&size=bestfit&width=435&height=101)