5: Sequential Reactions
- Page ID
- 23614
The propagation phase in a chain reaction is composed of a sequence of elementary reactions. The simple reduction pictured in Scheme 1 consists of two such reactions, and the radical addition shown in Scheme 2 has three elementary reactions in its propagation phase. Even though the overall reaction in each case (Schemes 1 and 2) consists of two or more elementary reactions occurring in sequence, neither overall reaction is described as a sequential one because to merit this designation, radical formation from the substrate and radical conversion to the product are not included.1 With these beginning and ending steps removed, the reactions shown in Schemes 1 and 2 do not qualify as sequential. [Cascade, domino, and tandem are other names used to describe sequential reactions.]
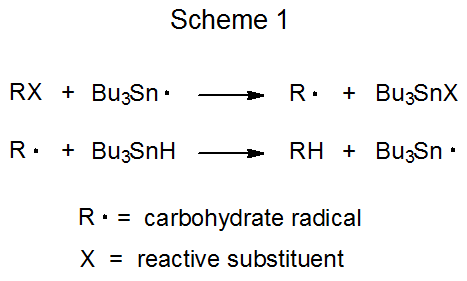
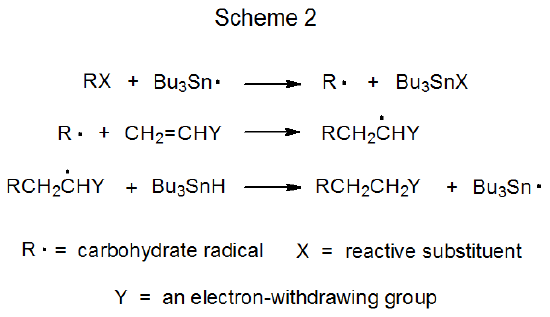
Elementary radical reactions readily occur in sequence because most produce a new radical poised for further reaction.1,2 Since all participants in a sequential reaction, including the intermediate radicals, are present in the reaction mixture at the same time, achieving the selectivity necessary to have each radical react in the desired manner at the correct time is a challenging task, one that is critical to the success of the overall process. This selectivity is controlled by the rates of the various, possible propagation steps.
Successful, sequential reactions have several common characteristics. Propagation steps are faster than termination steps. Intermediate radicals react rapidly with the correct nonradicals, but avoid reaction with the solvent and initiator. The final radical, but not the intermediate ones, is converted into a stable product.

