IV. Three-Step Sequential Reactions
- Page ID
- 23927
Three-step sequential reactions often consist primarily of combinations of cyclization and β-fragmentation steps.56,73–82 One group of such reactions involves ring opening of cyclic acetals.73–77 Since these reactions contain more fragmentation than cyclization, they tend to produce less complicated structures (e.g., compounds with fewer rings). In the reaction shown in Scheme 12, for example, internal radical addition to a cyano group creates a nitrogen-centered radical. This cyclization then is followed by successive β‑fragmentation steps. Together these elementary reactions cause acetal ring opening.73
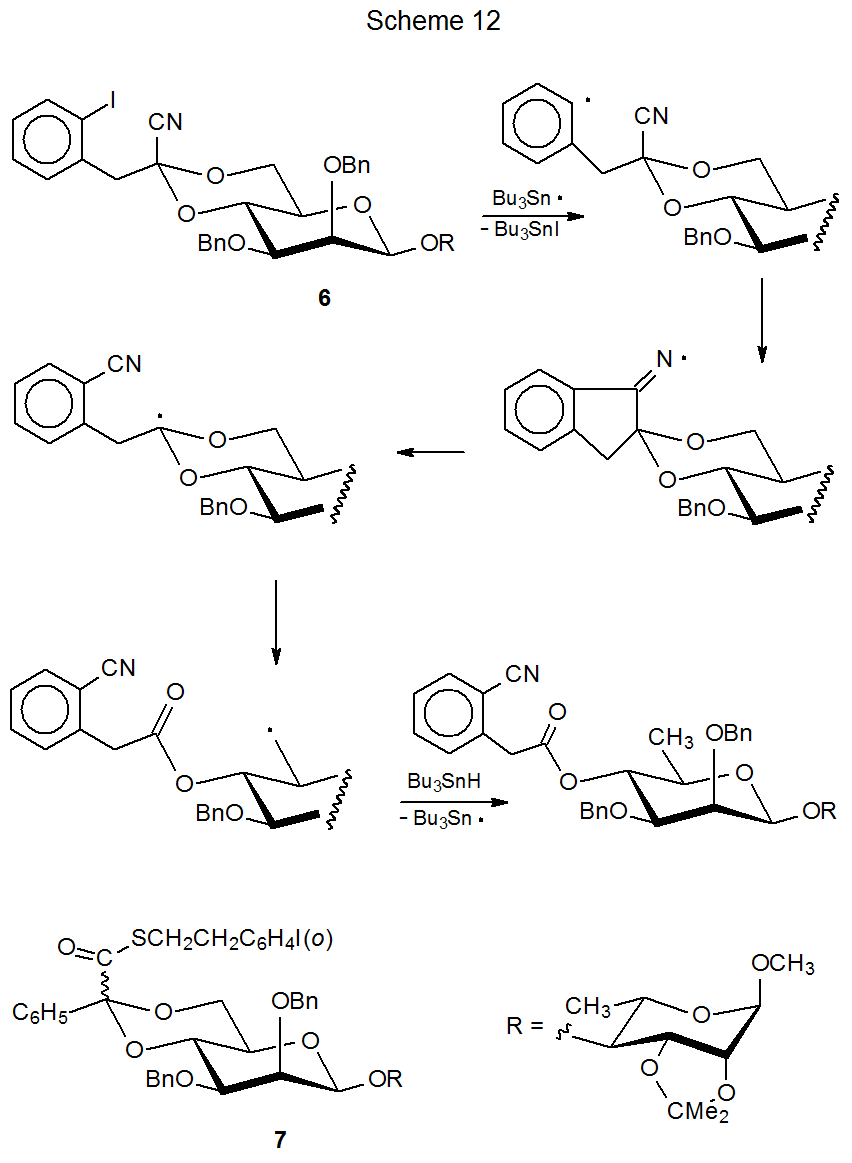
Balancing the advantage gained against additional effort needed to prepare the starting materials and adjust the reaction conditions is always a consideration when conducting a sequential reaction. Such consideration motivated the preparation of the acetal 6 (Scheme 12),73–75 when the effort necessary to synthesize 7, the first acetal used in this type of reaction, significantly offset the advantage derived from the sequential process.73,76,77
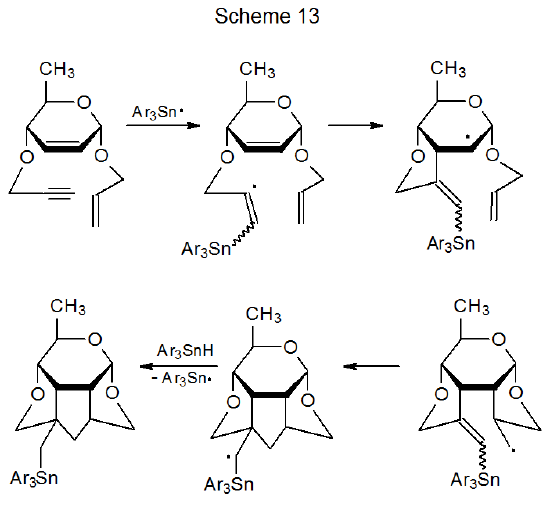
The synthetic potential of three-step sequential reactions changes when there is more cyclization than fragmentation. When such a change occurs, reactions can be used to build more complex structures.78–81 Specifically, the process shown in Scheme 13, which contains only cyclization steps, generates three new rings in a single reaction.81