I. Introduction
- Page ID
- 23941
A. The Evans-Polanyi Relation
For many radical reactions there is a simple relation between the energy of activation for the reaction and its enthalpy. This relation, which is referred to by several, similar names1–3 (Evans-Polanyi being a common one), is given in eq 1. Equation 1 expresses in a quantitative fashion the notion that in a group of closely related reactions the enthalpy for a particular reaction should be related to its energy of activation; specifically, energies of activation should decrease in a linear fashion as reactions become more exothermic.
.png?revision=1&size=bestfit&width=155&height=72)
Once the two constants in eq 1 have been determined, it is possible to predict the energy of activation for reaction of any member of the group from knowledge of the reaction enthalpy. The numerical value of the constant α represents the fraction of the overall enthalpy change that exists at the transition state. The value of α can be viewed as a measure of how far a reaction has proceeded along the reaction coordinate when the transition state is reached. The later a transition state occurs in a reaction the closer α will be to unity.
B. Nucleophilic and Electrophilic Radicals
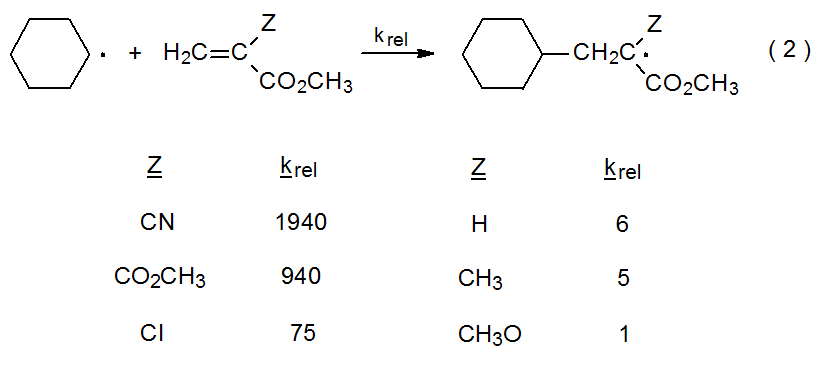
Although radicals are neutral species, they often exhibit behavior characteristic of either nucleophilic or electrophilic intermediates.4,5 This behavior facilitates certain types of reaction; for example, in the addition reactions shown in eq 2, the carbon-centered, cyclohexyl radical behaves as a nucleophile by adding more rapidly to compounds with more electron-deficient double bonds than to ones in which the double bonds are less electron-deficient.6 In contrast, the malonyl radical 1 can be viewed as electrophilic because it adds to electron-rich double bonds such as that in the D-glucal 2 (eq 3).7 A good beginning point for discussing radical philicity is examining some hydrogen-abstraction reactions.
.png?revision=1&size=bestfit&width=415&height=192)
.png?revision=1&size=bestfit&width=340&height=208)

