II. Bond Polarities, Bond-Dissociation Energies, and Rate Constants for Hydrogen-Abstraction Reactions
- Page ID
- 23935
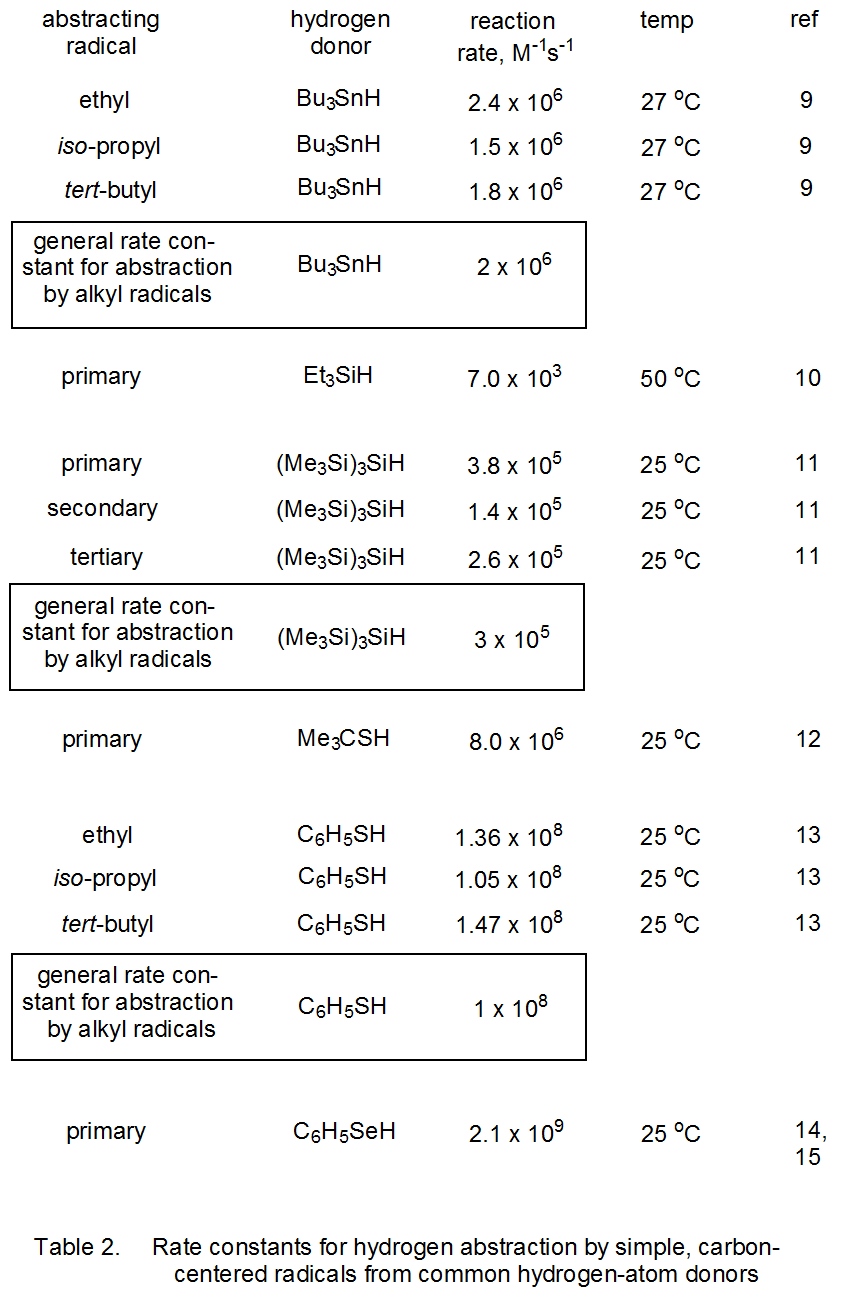
Bond polarities, bond-dissociation energies, and rate constants for abstraction of hydrogen atoms bonded to tin, silicon, sulfur, selenium, and carbon all are given in Table 1. Based on Pauling’s electronegativity values, hydrogen has a small negative charge when bonded to tin or silicon and a small positive charge when bonded to sulfur, selenium, or carbon. The information in Table 2 shows that for each type of bond, the rate constant for hydrogen-atom abstraction by simple primary, secondary, and tertiary, carbon-centered radicals is nearly the same.
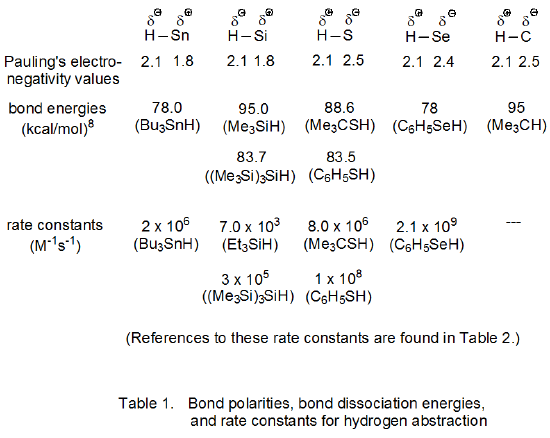
When the bond dissociation energies in Table 1 are used to calculate reaction enthalpies, they show that the reaction in eq 4 is more exothermic than that in eq 5. If the Evans-Polanyi relation is obeyed, the first reaction (eq 4) should have a lower energy of activation than the second (eq 5), but the opposite appears to be true. The rate constants for these reactions, when related to activation energies through the Arrhenius equation (eq 6), show that, unless the frequency factors for these two reactions are quite different, the reaction given in eq 4 actually has a higher energy of activation. Clearly, something in addition to reaction enthalpies must have a significant role in determining energies of activation for these two reactions.
.png?revision=1&size=bestfit&width=435&height=129)
.png?revision=1&size=bestfit&width=460&height=127)
.png?revision=1&size=bestfit&width=165&height=92)
The identification of a likely candidate for this additional factor can be made by returning to the reactions pictured in equations 2 and 3 and recalling that these reactions show some carbon-centered radicals to be nucleophilic and others electrophilic. If one assumes that the tert-butyl radical is similar in its philicity to the nucleophilic cyclohexyl radical, then in the reaction in eq 5 there is a polarity match between the nucleophilic radical and the electron-deficient hydrogen atom being abstracted. Since a similar match does not exist in the slower reaction (eq 4), radical philicity becomes a prime candidate for the factor that joins with reaction enthalpy in explaining the rate constants for hydrogen-abstraction reactions.

