III. Carbon-Centered Radicals
- Page ID
- 23948
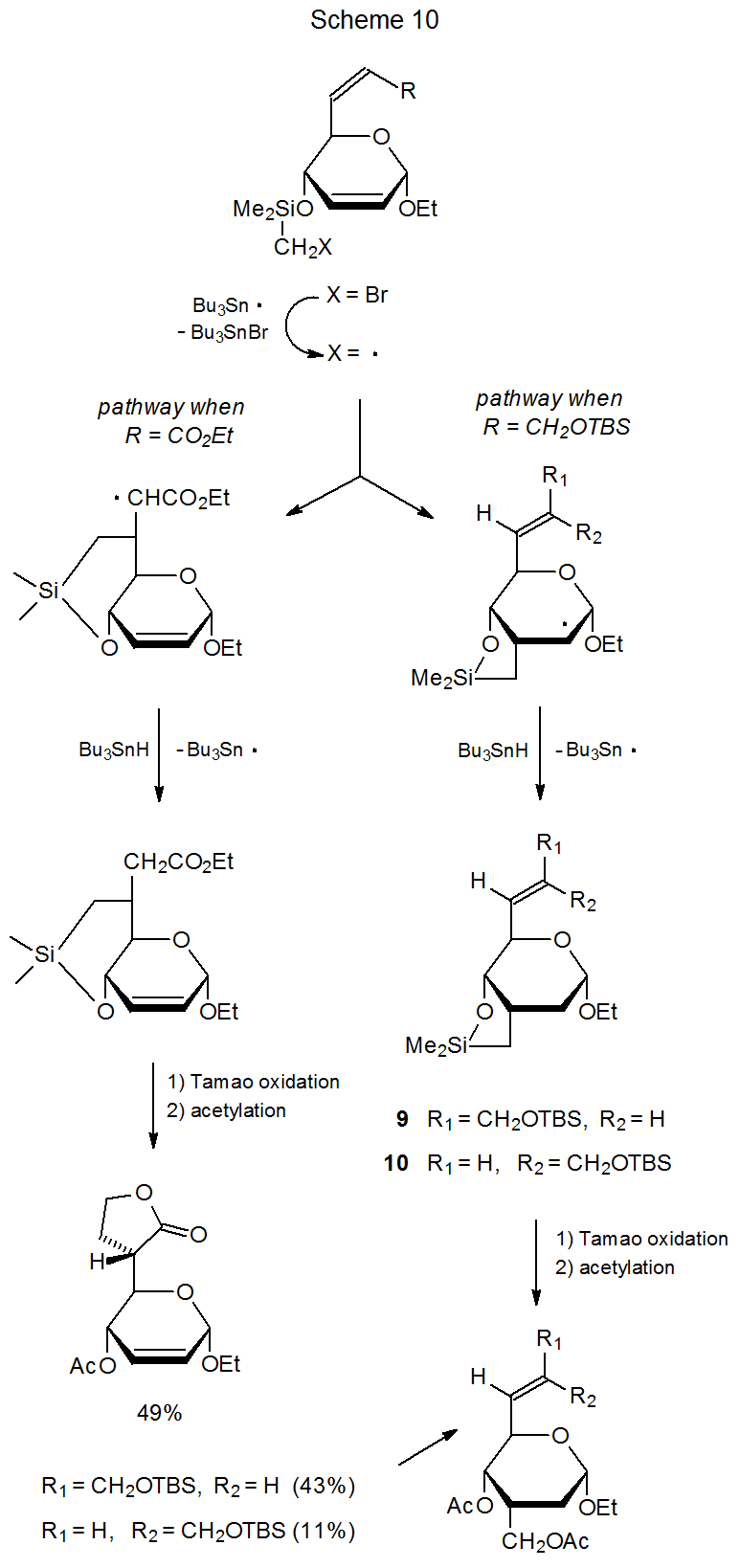
Although the primary reason for reacting carbohydrates with (Me3Si)3Si· or Bu3Sn· is to generate carbon-centered radicals, the chemoselectivity of the reactions of carbohydrate radicals formed in this way also is a matter of importance. Nearly all carbon-centered radicals derived from carbohydrates are nucleophilic and, consequently, add more readily to carbon–carbon multiple bonds that are electron deficient than to ones that are electron rich. (The rationale behind this selectivity is discussed in Sections IV.A.1. and IV.B.1. of Chapter 7.) The cyclization reactions shown in Scheme 10 illustrate the influence of an electronegative substituent on the chemoselectivity of addition of a nucleophilic radical to a compound with two double bonds.36,37 Reaction occurs at the double bond external to the ring when an electron-withdrawing substituent (R = CO2Et) is present, but if the substituent is not electron-withdrawing (R=CH2OTBS), addition is exclusively at the endocyclic double bond. (The cis-trans isomerization that takes place during formation of 9 and 10 results from reversible addition of Bu3Sn· to the double bond external to the ring.)
A variety of groups (e.g., acetal, acetoxy, carbonyl, cyano, hydroxy, and silyloxy groups) can be present in a reactant molecule but remain unchanged during carbon-centered radical addition to a carbon–carbon multiple bond. Carbon-centered radicals also participate less readily in substitution reactions with normally reactive substituents than do tin- and silicon-centered radicals; for example, Tables 1, 2, and 5 in Chapter 8 show the absolute rate constants for reaction of tert-butyl bromide with Bu3Sn·, (Me3Si)3Si·, and CH3(CH2)6CH2· are 1.4 x 108 M-1s-1, 1.2 x 108 M-1s-1, 4.6 x 103 M-1s-1, respectively. This reluctance to become involved in substitution reactions is illustrated in Scheme 2 where the radical 2 does not react with the chlorine atom or phenylseleno group in 3 but rather adds to the C–C double bond in this molecule.2

