I. Introduction
- Page ID
- 23950
A. Definitions of Regiospecific and Regioselective Reactions
When the terms regioselective and regiospecific were first introduced into organic chemistry, they were defined in the following way: “If a reaction proceeds without skeletal rearrangement to give exclusively (within experimental error) one of two or more possible isomers, it is called regiospecific. If there is a significant preponderance of one isomer formed, it is said to be regioselective.1" Since a regiospecific reaction can be viewed as a special type of regioselective reaction (i.e., one that is totally selective), the term regioselective can be used to describe any reaction that produces one structural isomer in greater abundance than another.
B. Regioselectivity in Radical Reactions of Carbohydrates
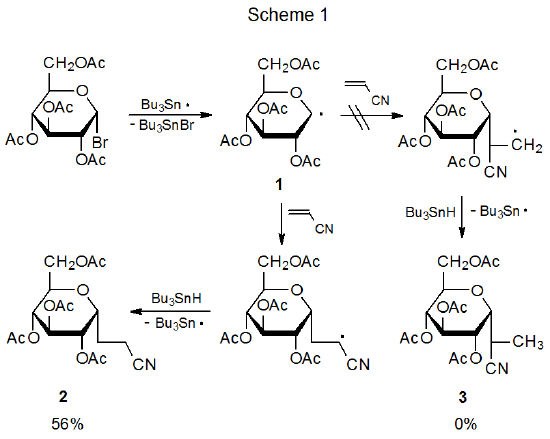
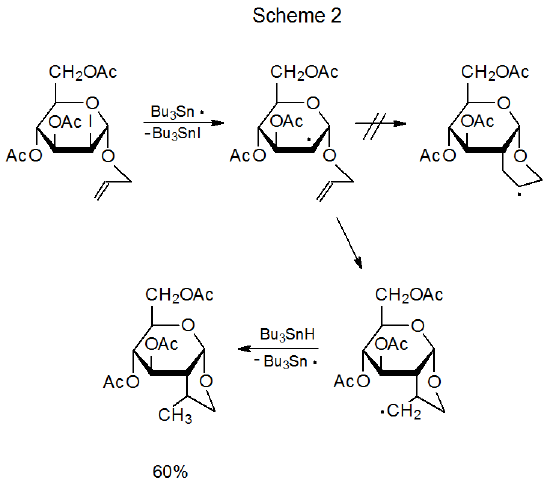
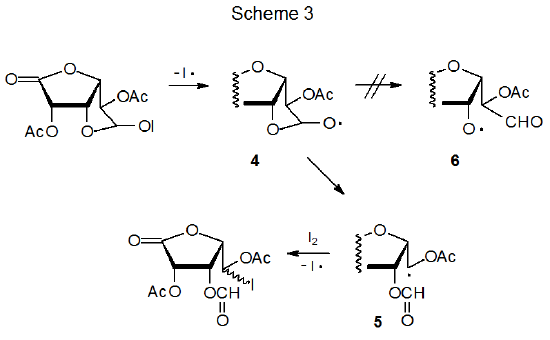
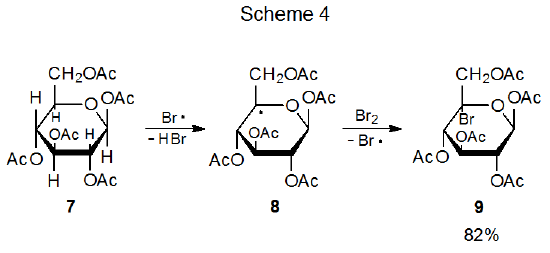
Among carbohydrates there are several types of radical reaction for which regioselectivity is an important consideration. The first of these (discussed in Section II) is the addition of a radical to the multiple bond in an unsaturated compound. An example of reaction of this type is found in Scheme 1, where addition of the pyranos-1-yl radical 1 takes place only at the unsubstituted carbon atom of the double bond in acrylonitrile.2,3 A second type of regioselective reaction (described in Section III) is a variation on this addition process that occurs when the radical center and the multiple bond are part of the same molecule. In the reaction shown in Scheme 2, for example, regioselectivity arises because there is a choice between producing a five-membered or six-membered ring.4 A third type of regioselective reaction, one involving β-fragmentation, is discussed in Section IV. An example is given in Scheme 3 where the oxygen-centered radical 4 undergoes ring opening to generate the carbon-centered radical 5 rather than the oxygen-centered radical 6.5 An example of the final type of regioselective radical reaction is found in Scheme 4, where abstraction of H-5 from the pentaacetate 7 takes place even though there are other hydrogen atoms present in 7 that could have been abstracted to form isomeric products.6 This type of regioselectivity, sometimes referred to as site-selectivity, is described in Section V.