II. Reaction Mechanisms
- Page ID
- 24009
A. Group Abstraction
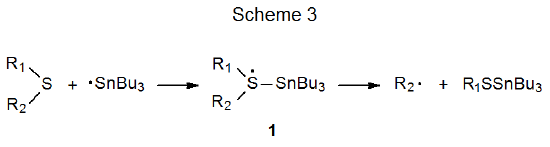
Two mechanisms are considered to be reasonable possibilities for the carbon–sulfur bond cleavage described by the reaction shown in eq 1.1 The first is the bimolecular, homolytic, substitution (SH2) reaction pictured in Scheme 2, and the second is a stepwise process that involves formation of an intermediate (1) with a hypervalent sulfur atom (Scheme 3). The choice between these two hinges on the existence of 1.

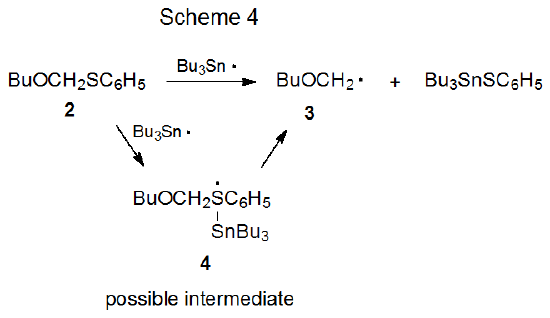
There is little experimental evidence upon which to base a decision about formation of a compound with a hypervalent sulfur atom during carbon–sulfur bond cleavage, but reaction of the thioacetal 2 with the tri-n-butyltin radical provides some suggestive information (Scheme 4).2 Although this reaction produces BuOCH2· (3), the effect of temperature on the ESR signal for this radical is unexpected because the intensity of the signal increases as the temperature in the ESR cavity rises. (Signals due to radicals arising from reaction of bromides with Bu3Sn· decrease with rising temperature due to leveling of the Boltzmann distribution of spin states.2) A possible explanation for this behavior is that a slow, temperature-dependent reaction between the thioacetal 2 and Bu3Sn· produces the hypervalent, sulfur-centered radical 4 (not observable by ESR), an intermediate that then fragments rapidly to give the ESR observable radical 3 (Scheme 4).2
Molecular orbital calculations also have been used to study the possibility of formation of intermediates with hypervalent sulfur atoms. When these calculations focus on the reactions of sulfides, they do no support the existence of such intermediates.1,3–6
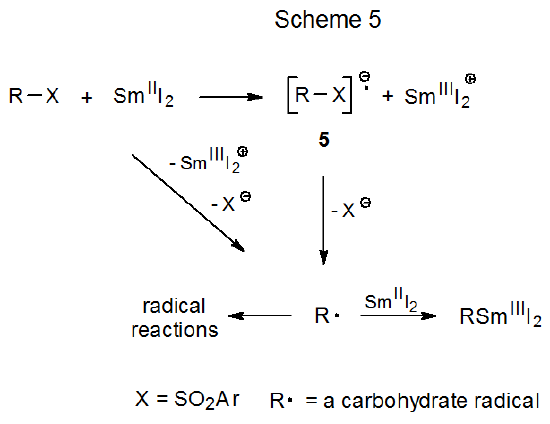
B. Electron-Transfer
Electron transfer to a sulfur-containing carbohydrate naturally depends upon such a compound having a group that readily accepts electrons. Sulfones meet this requirement and, thus, are prime candidates for electron-transfer reaction.7 Two proposed mechanisms showing how such transfer could lead to cleavage of a carbon–sulfur bond are shown in Scheme 5. In one of these a sulfone reacts with an electron donor (e.g., SmI2) to produce a radical anion (5) that then fragments to give an anion and a carbon-centered radical. In the other, dissociative electron transfer forms an anion and a carbon-centered radical directly from reaction of a sulfone with SmI2.