IV. Dithioacetals
- Page ID
- 24011
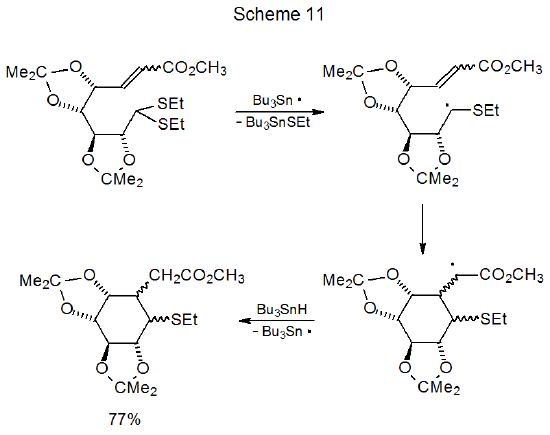
Dithioacetals react with tri-n-butyltin hydride to replace first one, and then the second, alkylthio group with a hydrogen atom (Scheme 10).30 Because the first group is replaced more rapidly than the second, good yields of compounds with a single sulfur atom are obtained under the proper reaction conditions.31–35 The greater reactivity of the first ethylthio group in these compounds is due to formation of an intermediate, carbon-centered radical that is stabilized by the sulfur atom in the remaining ethylthio group.
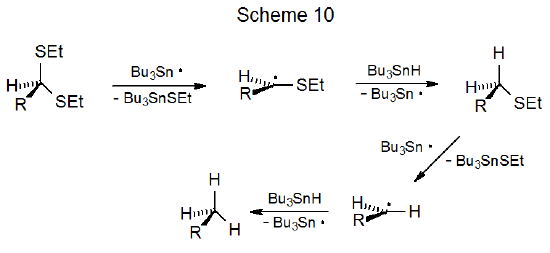
An unsaturated dithioacetal in which the double bond is properly positioned undergoes intramolecular radical addition.31–34 Reaction typically involves capture of the first-formed, carbon-centered radical by the multiple bond; thus, in the reaction shown in Scheme 11, the major product has a new ring system with an ethylthio substituent.31 Here again the greater reactivity of the first ethylthio group allows reaction to occur with no detectable loss of the second.