V. Thiocarbonates and Dithiocarbonates
- Page ID
- 24012
Thiocarbonates and dithiocarbonates are compounds in which at least one sulfur atom is bonded to the carbon atom of a carbonyl group. The reactivity of these compounds is similar to that of the sulfur-containing compounds already discussed in that reaction begins with carbon–sulfur bond cleavage producing the more stable of the possible carbon-centered radicals; thus, in the reaction shown in eq 5, product identity is consistent with forming an intermediate allylic radical from reaction of a thiocarbonate.36
.png?revision=2&size=bestfit&width=310&height=185)
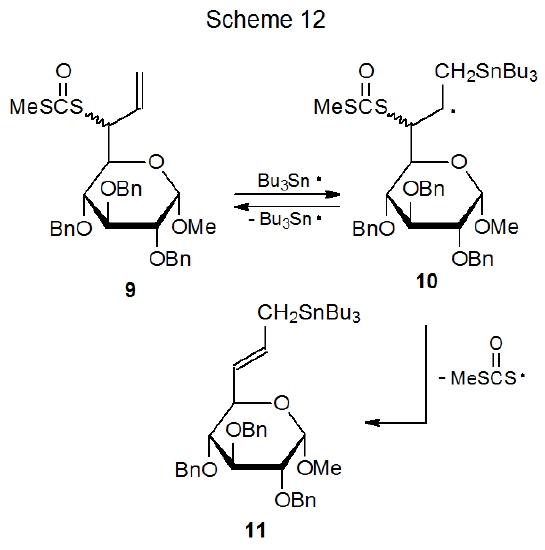
Addition of Bu3Sn· to the dithiocarbonate 9 is the first step in an addition-elimination reaction that produces the tin-containing compound 11 (Scheme 12).37 The stability of CH3SC(=O)S· is critical to this type of reaction because it, rather than Bu3Sn·, is expelled when a radical such as 10 forms a tin-containing product.37,38 Since Bu3Sn· addition to a double bond often is reversible, 10 sometimes may break a carbon–tin bond causing an undetectable regeneration of Bu3Sn· and the substrate 9.