VIII. Thiols and Thiyl Radicals
- Page ID
- 24015
In compounds with an H–S bond, hydrogen-atom abstraction to produce a sulfur-centered radical (eq 10) is a significant (sometimes the exclusive) reaction pathway. Such reactivity exists because thiols are among the most effective hydrogen-atom transfers in organic chemistry. Rate constants for hydrogen-atom abstraction by primary, secondary, and tertiary, carbon-centered radicals from thiophenol range from 0.8 x 108 to 1.5 x 108 M-1s-1 at 25 oC.70
.png?revision=1&size=bestfit&width=345&height=101)
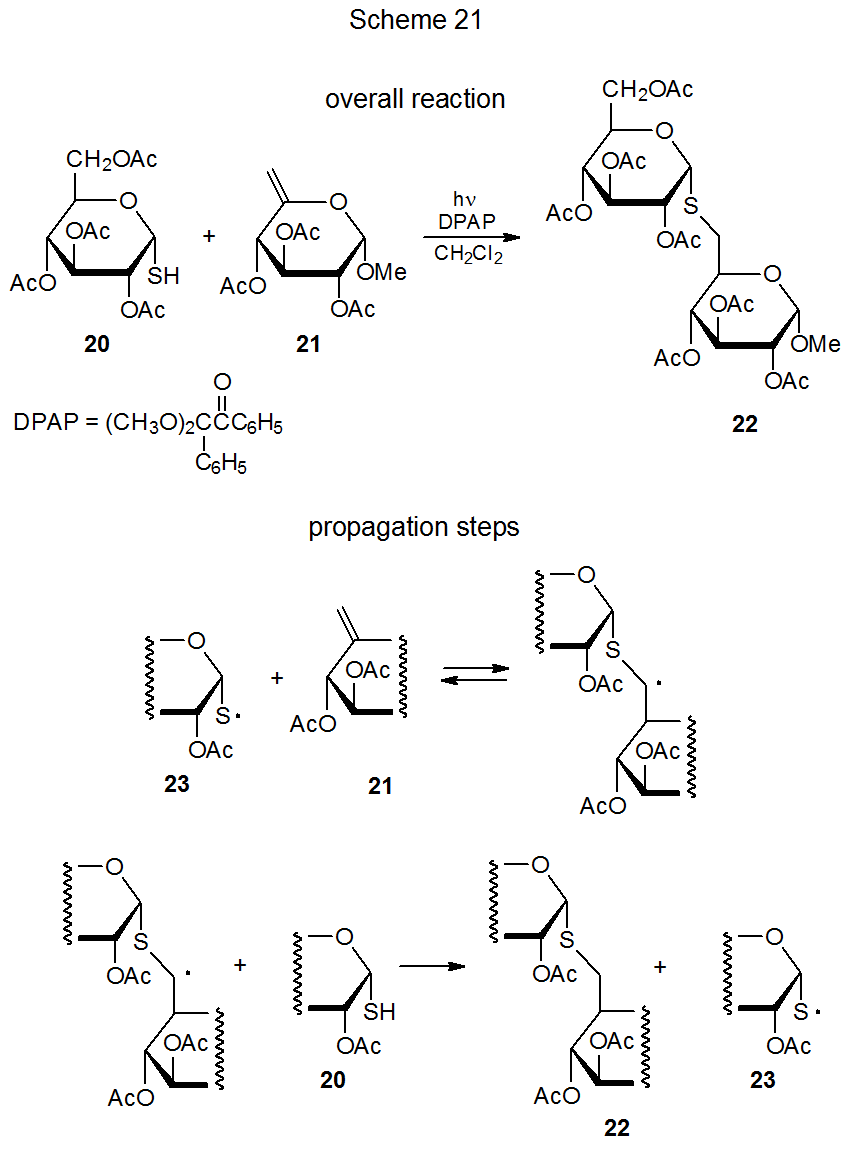
A characteristic reaction of a thiyl radical is addition to a carbon–carbon multiple bond.71–85 In the reaction shown in Scheme 21, for example, addition of the thiyl radical 23 to the unsaturated carbohydrate 21 leads to formation of the S-disaccharide 22.77 This reaction is not only regiospecific but hydrogen-atom abstraction from 20 is so much faster than reaction with the molecular oxygen dissolved in the reaction mixture that an inert atmosphere is not required for successful S-disaccharide formation. Similar radical addition takes place between the thiol 20 and various D-glycals, including the D-glucal 24 (eq 11).78
.png?revision=1&size=bestfit&width=435&height=197)
Even though the most common radical reaction of a compound with an H–S bond is hydrogen-atom abstraction, under some conditions the HS group is replaced by a hydrogen atom (eq 12).86
.png?revision=1&size=bestfit&width=430&height=129)
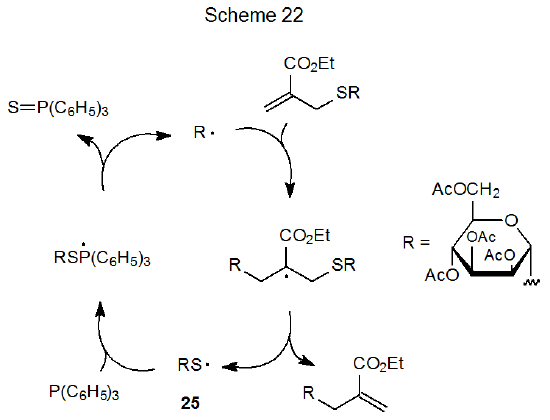
Although a carbohydrate containing a sulfur-centered radical typically is generated by hydrogen-atom abstraction from a thiol, the reaction shown in eq 13 forms a thiyl radical by the addition-elimination sequence pictured in Scheme 22.87 Critical to chain propagation in this reaction is the removal of the sulfur atom from 25 by reaction with triphenylphosphine.
.png?revision=1&size=bestfit&width=425&height=109)